Answered step by step
Verified Expert Solution
Question
1 Approved Answer
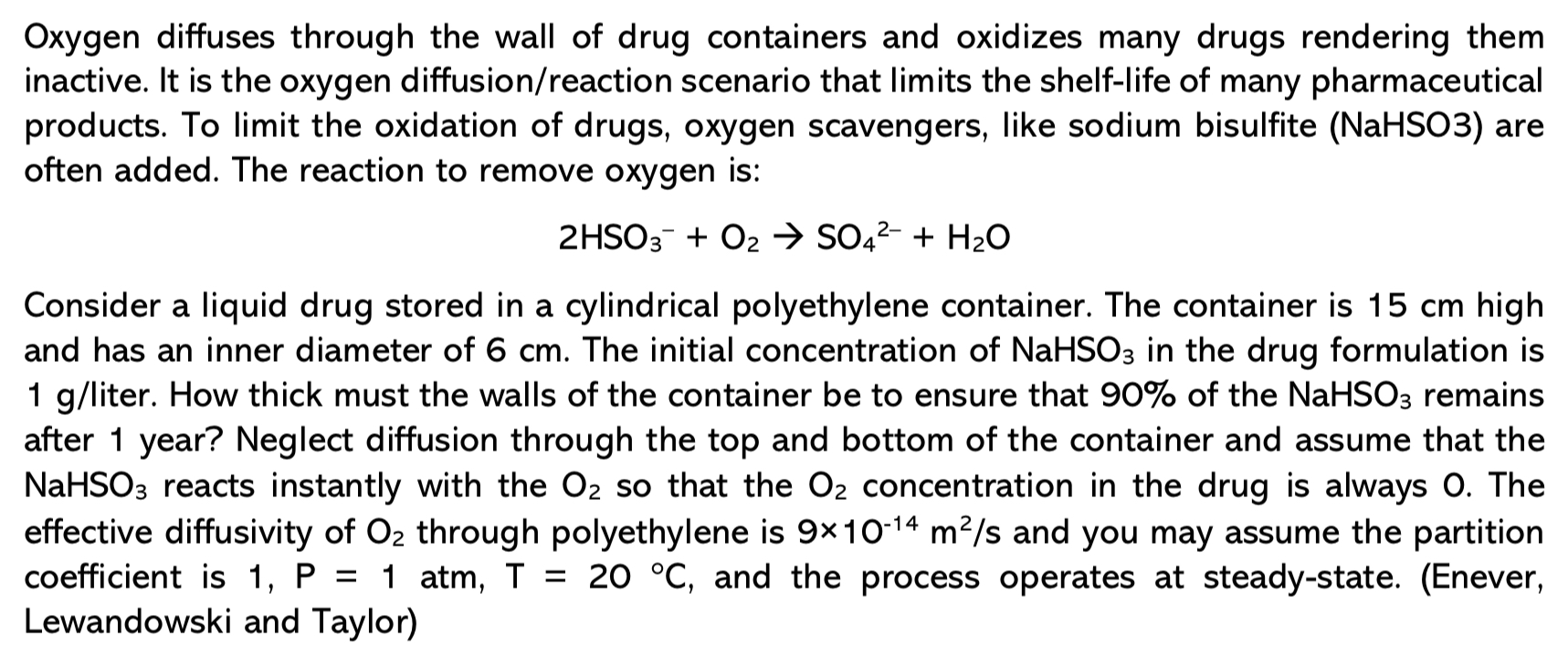
Oxygen diffuses through the wall of drug containers and oxidizes many drugs rendering them inactive. It is the oxygen diffusion / reaction scenario that limits
Oxygen diffuses through the wall of drug containers and oxidizes many drugs rendering them
inactive. It is the oxygen diffusionreaction scenario that limits the shelflife of many pharmaceutical
products. To limit the oxidation of drugs, oxygen scavengers, like sodium bisulfite NaHSO are
often added. The reaction to remove oxygen is:
Consider a liquid drug stored in a cylindrical polyethylene container. The container is high
and has an inner diameter of The initial concentration of in the drug formulation is
liter. How thick must the walls of the container be to ensure that of the remains
after year? Neglect diffusion through the top and bottom of the container and assume that the
reacts instantly with the so that the concentration in the drug is always The
effective diffusivity of through polyethylene is and you may assume the partition
coefficient is atm, and the process operates at steadystate. Enever
Lewandowski and Taylor
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


