Answered step by step
Verified Expert Solution
Question
1 Approved Answer
P 1 1 . 2 Assessing the Speciation of Bromomethane and of Atrazine in a QuartzAssessing the Speciation of Bromomethane and Atrazine in Quartz Sand
P Assessing the Speciation of Bromomethane and of Atrazine in a QuartzAssessing the Speciation of Bromomethane and Atrazine in Quartz Sand
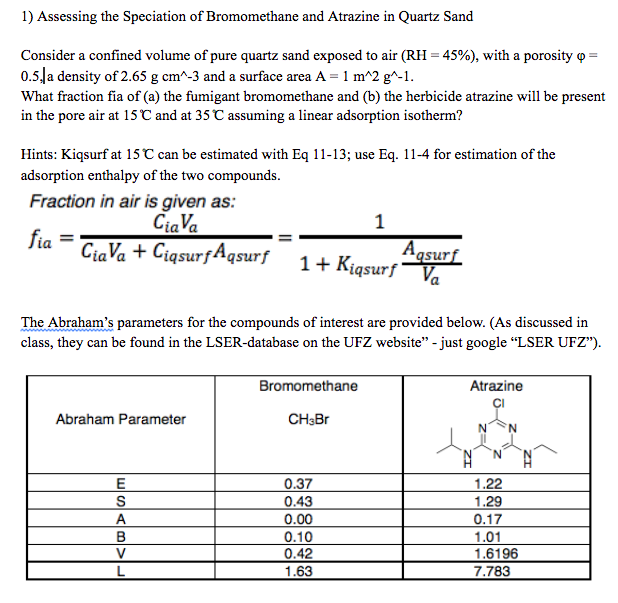
Consider a confined volume of pure quartz sand exposed to air with a porosity
a density of and a surface area
What fraction fia of a the fumigant bromomethane and b the herbicide atrazine will be present
in the pore air at and at assuming a linear adsorption isotherm?
Hints: Kiqsurf at can be estimated with Eq ; use Eq for estimation of the
adsorption enthalpy of the two compounds.
Fraction in air is given as:
The Abraham's parameters for the compounds of interest are provided below. As discussed in
class, they can be found in the LSERdatabase on the UFZ website" just google "LSER UFZ"
Sand
Consider a con!ned volume of pure quartz sand exposed to air RH with
a porosity see Box a density of g cm and a surface area A
m g What fraction fia of a the fumigant bromomethane and b the herbicide
atrazine will be present in the pore air at Cand at Cassuminga linear adsorption
isotherm? Comment on the result.
Hint: Consult Box Use Eq for estimation of the adsorption enthalpy of the
two compounds.
log KiqsurfaKRH mLi Ai Bi
and
log KiqsurfaKRH mLi Ai Bi
deltasurfaHikJ mol logKisurfam
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


