Answered step by step
Verified Expert Solution
Question
1 Approved Answer
P 2 - 6 B B The exothermic reaction AlongrightarrowB + C was carried out adiabatically and the following data recorded: The entering molar flow
P The exothermic reaction AlongrightarrowB was carried out adiabatically and the following data recorded: The entering molar flow rate of A was a What are the PFR and CSTR volumes necessary to achieve conver sion? b Over what range of conversions would the CSTR and PFR reactor vol umes be identical? c What is the maximum conversion that can be achieved in a CSTR d What conversion can be achieved if a dm PFR is followed in series by a CSTR e What conversion can be achieved if a CSTR is followed in a series by a PFR f Plot ihe conversion and rate of reaction as a function of PFR reactor vol ume up to a volume of P In a certain chemical plant, a reversible fluidphase isomerization is carried out over a solid catalyst in a tubular packedbed reactor. If the reac tion is so rapid that mass transfer between the catalyst surface and the bulk fluid is ratelimiting show that the kinetics are described in terms of the bulk concentrations and by where moles of reacting per unit area catalyst per unit time mass transfer coefficients for A and reaction equilibrium constant It is desired to double the capacity of the existing plant by processing twice the feed of reactant A while maintaining the same fractional conversion of to in the reactor. How much larger a reactor, in terms of catalyst weight, would be required if all other operating variables are held constant? You may use the ThoenesKramers correlation for mass transfer coefficients in a packed bed. Describe the effects of the flow rate, temperature, particle size at conversion. 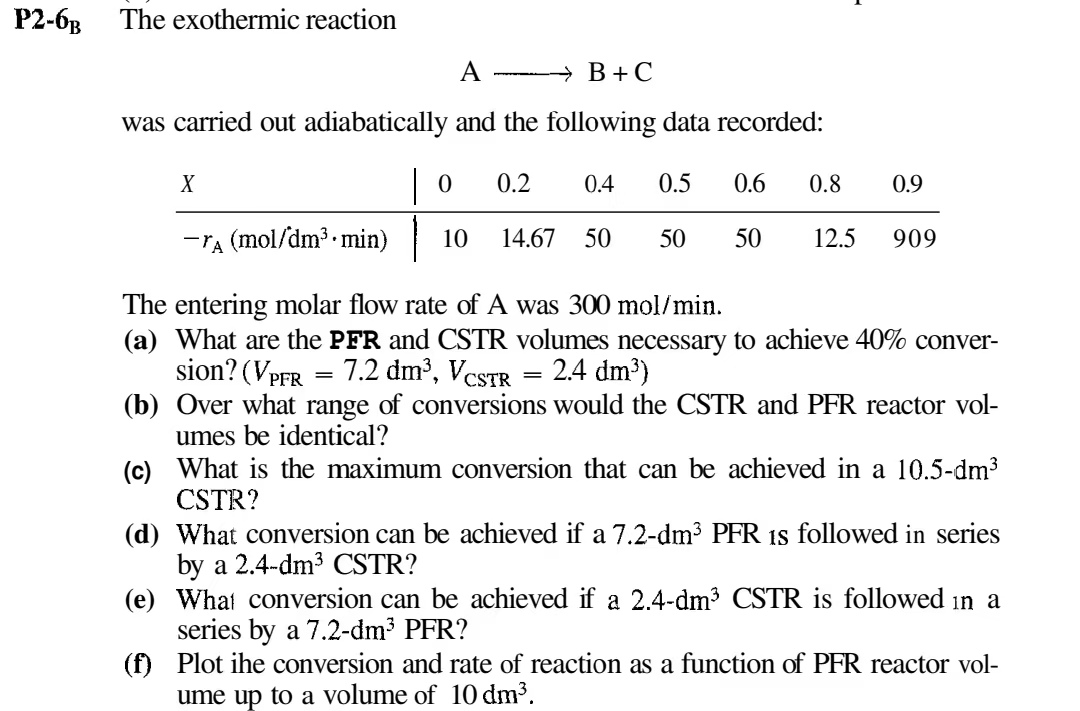
P The exothermic reaction
AlongrightarrowB
was carried out adiabatically and the following data recorded:
The entering molar flow rate of A was
a What are the PFR and CSTR volumes necessary to achieve conver
sion?
b Over what range of conversions would the CSTR and PFR reactor vol
umes be identical?
c What is the maximum conversion that can be achieved in a
CSTR
d What conversion can be achieved if a dm PFR is followed in series
by a CSTR
e What conversion can be achieved if a CSTR is followed in a
series by a PFR
f Plot ihe conversion and rate of reaction as a function of PFR reactor vol
ume up to a volume of P In a certain chemical plant, a reversible fluidphase isomerization
is carried out over a solid catalyst in a tubular packedbed reactor. If the reac
tion is so rapid that mass transfer between the catalyst surface and the bulk
fluid is ratelimiting show that the kinetics are described in terms of the bulk
concentrations and by
where moles of reacting per unit area catalyst per unit time
mass transfer coefficients for A and
reaction equilibrium constant
It is desired to double the capacity of the existing plant by processing twice
the feed of reactant A while maintaining the same fractional conversion of
to in the reactor. How much larger a reactor, in terms of catalyst weight, would
be required if all other operating variables are held constant? You may use the
ThoenesKramers correlation for mass transfer coefficients in a packed bed.
Describe the effects of the flow rate, temperature, particle size at conversion.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


