Answered step by step
Verified Expert Solution
Question
1 Approved Answer
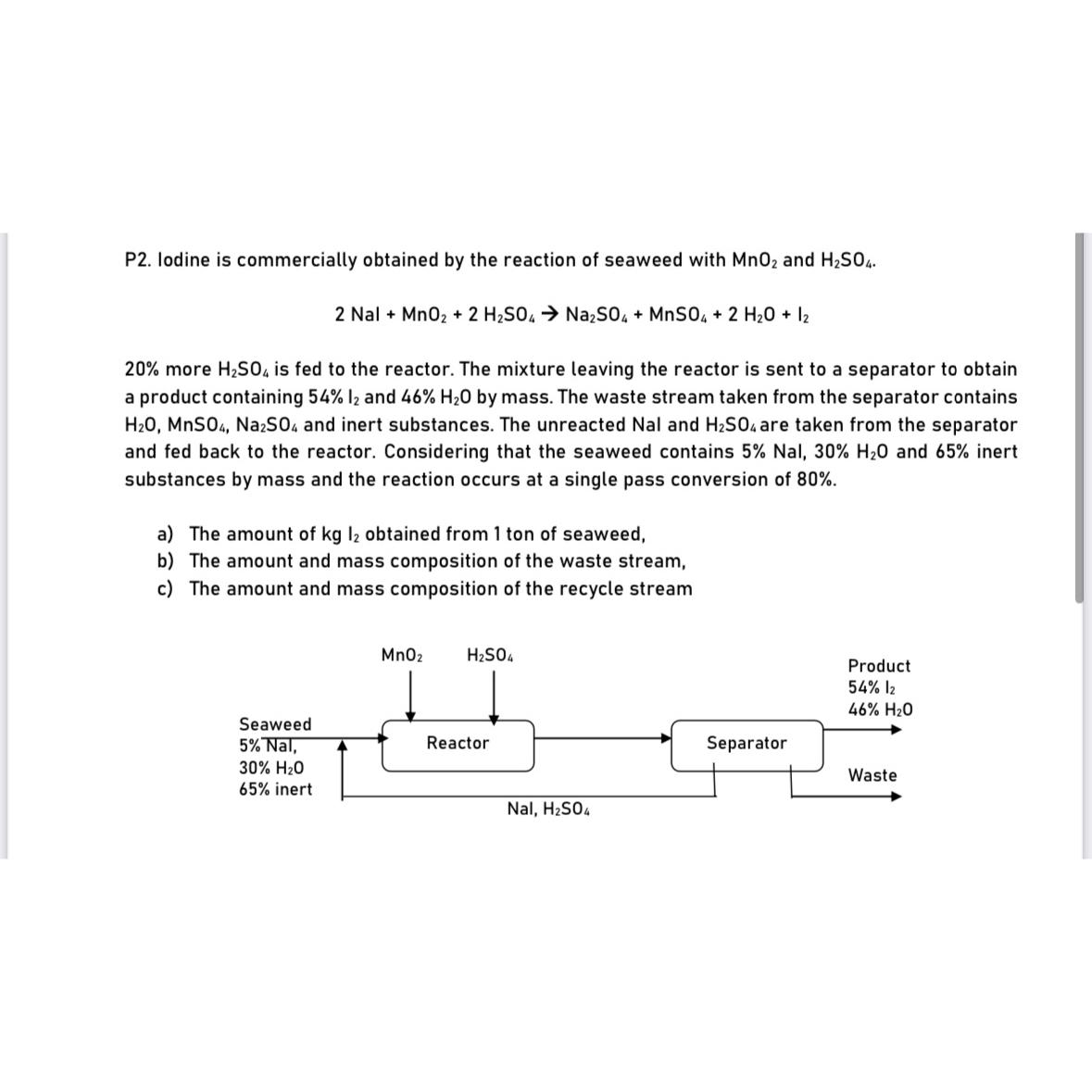
P 2 . lodine is commercially obtained by the reaction of seaweed with M n O 2 and H 2 S O 4 . 2
P lodine is commercially obtained by the reaction of seaweed with and
Nal
more is fed to the reactor. The mixture leaving the reactor is sent to a separator to obtain a product containing and by mass. The waste stream taken from the separator contains and inert substances. The unreacted Nal and are taken from the separator and fed back to the reactor. Considering that the seaweed contains Nal, and inert substances by mass and the reaction occurs at a single pass conversion of
a The amount of obtained from ton of seaweed,
b The amount and mass composition of the waste stream,
c The amount and mass composition of the recycle stream
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


