Answered step by step
Verified Expert Solution
Question
1 Approved Answer
P 7 - 6 ? B OEQ ( Old Exam Question ) . The liquid - phase irreversible reaction A B + C is carried
P OEQ Old Exam Question The liquidphase irreversible reaction is carried out in a CSTR To leam the rate law, the volumetric flow rate, hence is varied and the effluent concentrations of species A are recorded as a function of the space time Pure A enters the reactor at a concentration of Steadystate conditions exist when the measurements are recorded, tableRun a Determine the reaction order and specific reactionrate constant. b What experiments would you do to determine the activation energy? C If you were to take more data, where would you place the measurements d It is believed that the technician may have made a dilution factorof error in one of the concentration measurements. What do you think? Note: All measurements were taken at steadystate conditions. Be careful; this problem is sneaky. 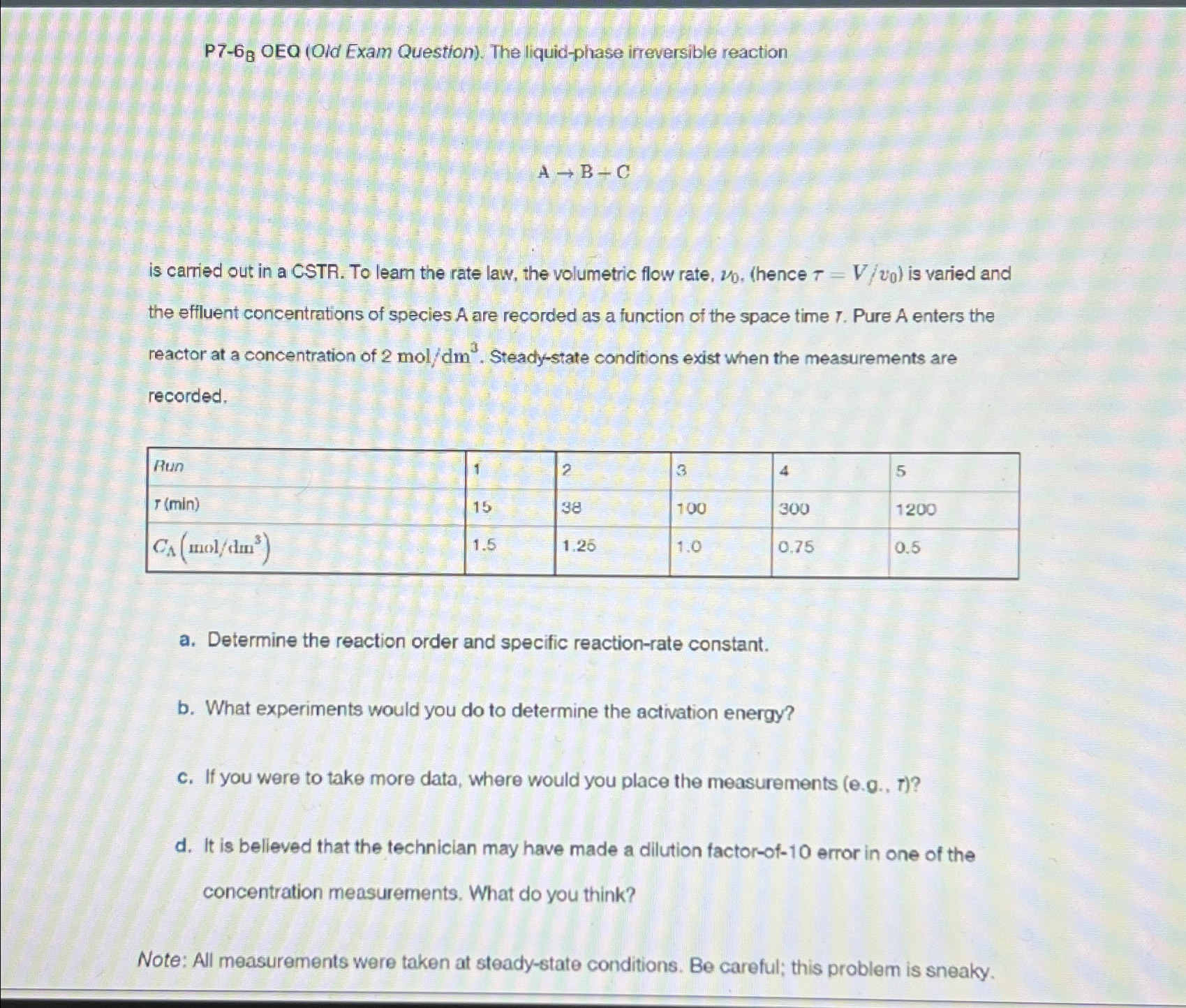
P OEQ Old Exam Question The liquidphase irreversible reaction
is carried out in a CSTR To leam the rate law, the volumetric flow rate,
hence is varied and the effluent concentrations of species A are recorded as a function of the space time Pure A enters the reactor at a concentration of Steadystate conditions exist when the measurements are recorded,
tableRun
a Determine the reaction order and specific reactionrate constant.
b What experiments would you do to determine the activation energy?
C If you were to take more data, where would you place the measurements
d It is believed that the technician may have made a dilution factorof error in one of the concentration measurements. What do you think?
Note: All measurements were taken at steadystate conditions. Be careful; this problem is sneaky.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access with AI-Powered Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started

