Answered step by step
Verified Expert Solution
Question
1 Approved Answer
P17F.9* J. Czarnowski and H.J. Schuhmacher (Chem. Phys. Lett. 17, 235 (1972)) suggested the following mechanism for the thermal decomposition of F,O in the
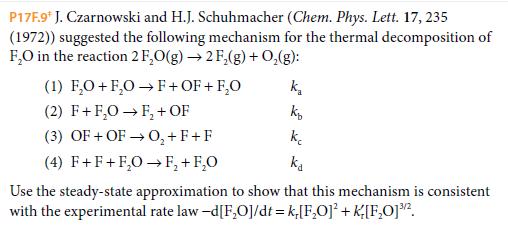
P17F.9* J. Czarnowski and H.J. Schuhmacher (Chem. Phys. Lett. 17, 235 (1972)) suggested the following mechanism for the thermal decomposition of F,O in the reaction 2 F,O(g) 2 F,(g) + 0,(g): (1) F,O+ F,O F+ OF + F,O k, (2) F+F,0 F, + OF (3) OF + OF 0,+ F+F (4) F+F+F,0 F, + F,0 ke ka Use the steady-state approximation to show that this mechanism is consistent with the experimental rate law -d[F,O]/dt = k,[F,O] + K[F,O]2.
Step by Step Solution
★★★★★
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


