The RiceHerzfeld mechanism for the thermal decomposition of acetaldehyde (CH 3 CO(g)) is Using the steady-state approximation,
Question:
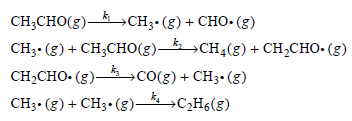
Using the steady-state approximation, determine the rate of methane (CH4(g)) formation.
Transcribed Image Text:
CH CHO(3) — CHз- (3) + CHO- (8) CH- (g) + CH,CHо(з) — сн4(8) + сH-CнO-(3) CH2CHO- (g)– CH- (8) + CHз-(g)—сН6(8) —со() + CHз- (8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
The differential rate expressions for methane and relevant intermediate species ...View the full answer

Answered By

Shebla K
I am an MBA graduate having experience as an Assistant Professor at University level for two years. I always prepare well for a class as I believe that only if you become an ocean you can give a bucket of water. Being a teacher was not only my profession but also my passion.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the reaction 2A + B products a friend proposes the following mechanism: A + B M A + M products a. Assuming that the second step is the rate-determining step and the first step is a fast...
-
Because of its importance in atmospheric chemistry, the thermal decomposition of nitric oxide, 2 NO (g) -7 N,(g) + O,(g), has been amongst the most thoroughly studied of gas-phase reactions. The...
-
The thermal decomposition of nitryl chloride, NO2Cl, 2NO2Cl(g) 2NO2(g) + Cl2(g) is thought to occur by the mechanism shown in the following equations: What rate law is predicted by this mechanism?...
-
Harry Bhel carries a business as a sole proprietorship. During its 2022 fiscal period, its first year of operations, the business had cash sales of $123,000. It also has sales on account of $46,000,...
-
Find the zeros of the function algebraically. 1. f(x) = 5x2 + 4x - 1 2. f(x) = 8x + 3/11 - x
-
What is the surface area of a cube if volume is 729 cm 3 ?
-
Describe and give the advantages and disadvantages of (a) moving averages and (b) exponential smoothing. LO.1
-
(Deferred Tax Asset with Previous Valuation Account) Assume the same information as E19-14, except that at the end of 2010, Callaway Corp. had a valuation account related to its deferred tax asset of...
-
Metlock inc Question 25 Metlock, Inc. just began business and made the following four inventory purchases in June June 1 135 units $890 June 10 180 units 1208 June 15 180 units 1232 June 28 135 units...
-
a. Assume that you are called in as a forensic accountant to advise Geox. How would you suggest evaluating the contemplated open-source shopping cart systems? b. How does the fact that the systems...
-
In the troposphere carbon monoxide and nitrogen dioxide undergo the following reaction: NO 2 (g) + CO(g) NO(g) + CO 2 (g) Experimentally, the rate law for the reaction is second order in NO 2 (g),...
-
Reciprocal plots provide a relatively straightforward way to determine if an enzyme demonstrates Michaelis Menten kinetics and to determine the corresponding kinetic parameters. However, the slope...
-
Discuss the three steps in determining cost of sales in a periodic inventory system.
-
Part 1 of 4 05 points abook Print References Required information Problem 24-2A (Algo) Payback period, accounting rate of return, net present value, and net cash flow calculation LO P1, P2, P3 [The...
-
Keenan Music's CEO has been pondering about the recent proposal of the Specialty Guitar Project. The accountant has done a capital budgeting analysis on the project and outlined the conditions that...
-
On July 1, 2025, Sheridan Co. pays $15,000 to Blue Spruce Insurance Co. for a 2-year insurance contract. Both companies have fiscal years ending December 31. (a1) Journalize the entry on July 1 and...
-
A CU triaxial test with c = 20 psi is performed on a sand and a deviator stress of 80 psi fails the specimen. Previous tests revealed that the effective friction angle for this sand is 35. Calculate...
-
Haliburton Mills Inc. is a large producer of men's and women's clothing. The company uses standard costs for all of its products. The standard costs and actual costs for a recent period are given...
-
Suppose that the airplane in FIGURE 3.97 is flying at 0.2 kilometer per second to the left, rather than to the right. If the position of the airplane is fixed at (-1, 5), graph the image of the...
-
Smthe Co. makes furniture. The following data are taken from its production plans for the year. Required: 1. Determine the hazardous waste disposal cost per unit for chairs and for tables if costs...
-
A sample of propane (C 3 H 8 ) is placed in a closed vessel together with an amount of O 2 that is 2.15 times the amount needed to completely oxidize the propane to CO 2 and H 2 O at constant...
-
Propose a structure for a compound with molecular formula C 10 H 14 O that exhibits the following 1 H NMR spectrum. Proton NMR Chemical shift (ppm)
-
A van der Waals gas undergoes an isothermal reversible expansion under conditions such that z < 1. Is the work done more or less than if the gas followed the ideal gas law?
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App


