Question
P7.13 A mixture of 35 mol% ethanol/65 mol% water is equilibrated at 1 atm and 86C. Using Fig. 7.7, determine y, XE, and the
![]()
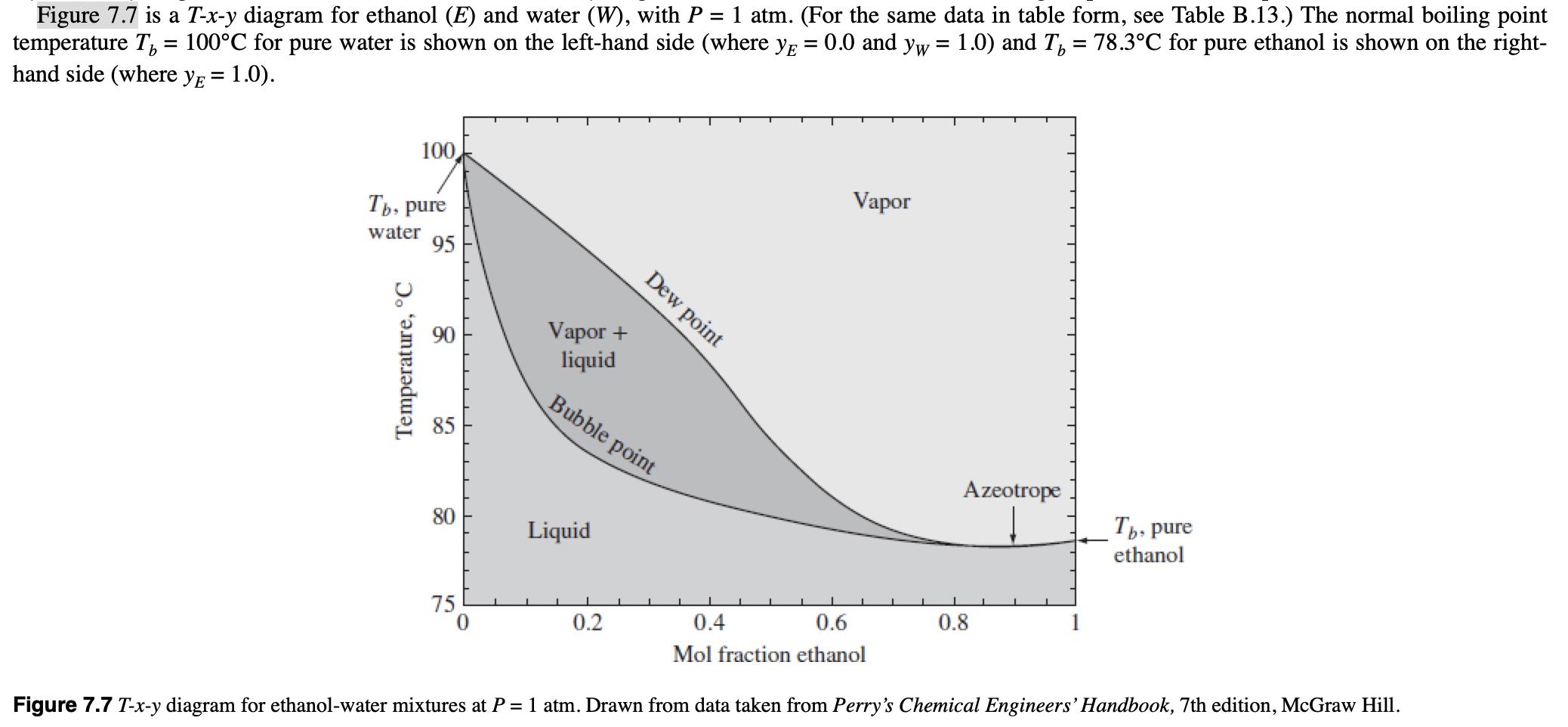
P7.13 A mixture of 35 mol% ethanol/65 mol% water is equilibrated at 1 atm and 86C. Using Fig. 7.7, determine y, XE, and the fraction of the mixture that is vaporized. Figure 7.7 is a T-x-y diagram for ethanol (E) and water (W), with P = 1 atm. (For the same data in table form, see Table B.13.) The normal boiling point temperature T = 100C for pure water is shown on the left-hand side (where y = 0.0 and yw = 1.0) and T = 78.3C for pure ethanol is shown on the right- hand side (where y = 1.0). 100 Tb, pure water 95 95 emperature, C 888 85 90 Vapor + liquid Dew point Bubble point 60 80 Liquid Vapor Azeotrope Tb, pure ethanol 75 0 0.2 0.4 0.6 Mol fraction ethanol 0.8 Figure 7.7 T-x-y diagram for ethanol-water mixtures at P = 1 atm. Drawn from data taken from Perry's Chemical Engineers' Handbook, 7th edition, McGraw Hill.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
4th edition
978-1118431221, 9781119192138, 1118431227, 1119192137, 978-1119498759
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



