Question
Sucrose is hydrolysed enzymatically to glucose and fructose in the human body. This reaction can also be carried out industrially with strong acid catalysis
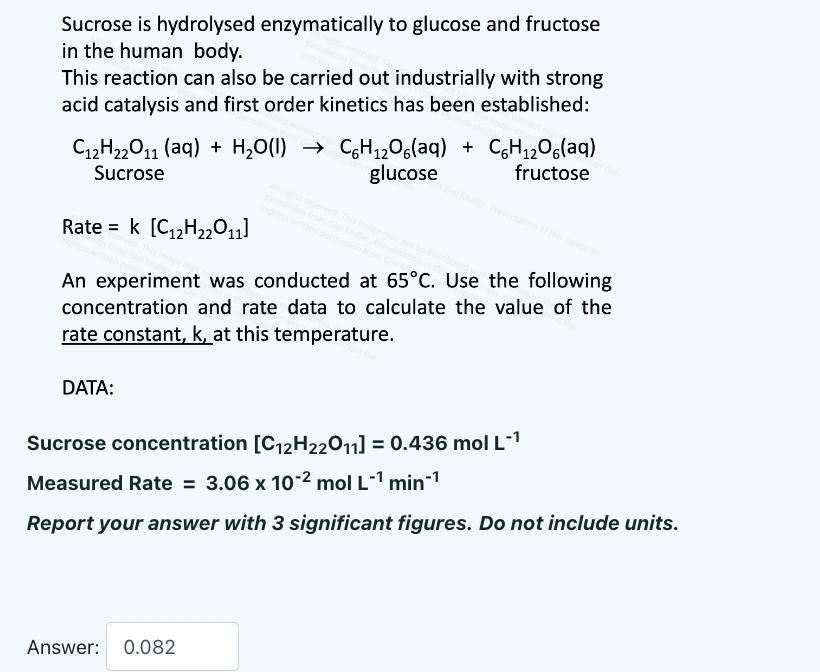
Sucrose is hydrolysed enzymatically to glucose and fructose in the human body. This reaction can also be carried out industrially with strong acid catalysis and first order kinetics has been established: C2H22O11 (aq) + HO(1) C6H2O6(aq) + C6H2O6(aq) Sucrose glucose fructose Ratek [C2H201] An experiment was conducted at 65C. Use the following concentration and rate data to calculate the value of the rate constant, k, at this temperature. DATA: Sucrose concentration [C2H22011] = 0.436 mol L- Measured Rate = 3.06 x 10-2 mol L-1 min- Report your answer with 3 significant figures. Do not include units. Answer: 0.082
Step by Step Solution
3.48 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
Solution Rate 2 306x ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Accounting
Authors: Lew Edwards, John Medlin, Keryn Chalmers, Andreas Hellmann, Claire Beattie, Jodie Maxfield, John Hoggett
9th edition
1118608224, 1118608227, 730323994, 9780730323990, 730319172, 9780730319177, 978-1118608227
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



