Question: Part 1 (1 point) Remember to use square brackets to show concentration. Do not include multiplication symbols in the rate equation. Do not places



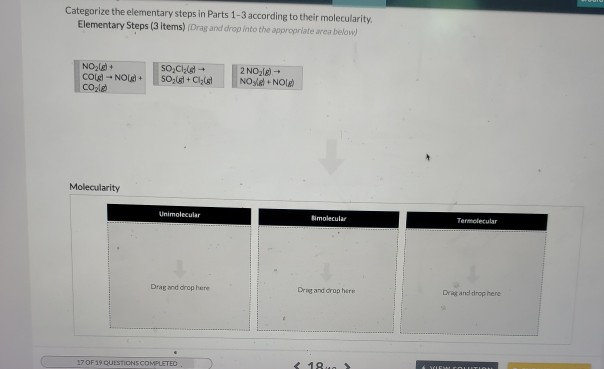
Part 1 (1 point) Remember to use square brackets to show concentration. Do not include multiplication symbols in the rate equation. Do not places spaces in your answer. See Hint Write the rate law for the following reaction, which represents an elementary step in a reaction. Your rate law should not include the states of matter. socl(g) SO(g) + Cl(g) Rate= Part 2 (1 point) Remember to use square brackets to show concentration. Do not include multiplication symbols in the rate equation. Do not place spaces in your answer. See Hint Write the rate law for the following reaction, which represents an elementary step in a reaction. Your rate law should not include the states of matter. NO(g) + CO(g) - NO(g) + CO(g) Rate 8. Part 3 (1 point) Remember to use square brackets to show concentration. Do not include multiplication symbols in the rate equation. Do not place spaces in your answer. Write the rate law for the following reaction, which represents an elementary step in a reaction. Your rate law should not include the states of matter. 2NO(g) NO(g) + NO(g) Rate- See Hint - Categorize the elementary steps in Parts 1-3 according to their molecularity. Elementary Steps (3 items) (Drag and drop into the appropriate area below) NO(g) + CO-NO+ CO) Molecularity SOCl SO + Clst Unimolecular Drag and drop here. 17 OF 19 QUESTIONS COMPLETED 2 NO(g) NO+NO(g) Bimolecular Drag and drop here < 18 > Termolecular Drag and drop here &VIEW COLUTION
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
part1 ratekSO 2 Cl 2 part2 ratek... View full answer

Get step-by-step solutions from verified subject matter experts


