Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part 1: First Reaction (NaOH + HCI) NaOH(aq) + HCl(aq) HO(1) + NaCl(aq) Part 2: Second Reaction (NaOH + NH4CI) NaOH(aq) + NHCl(aq) NH(aq)
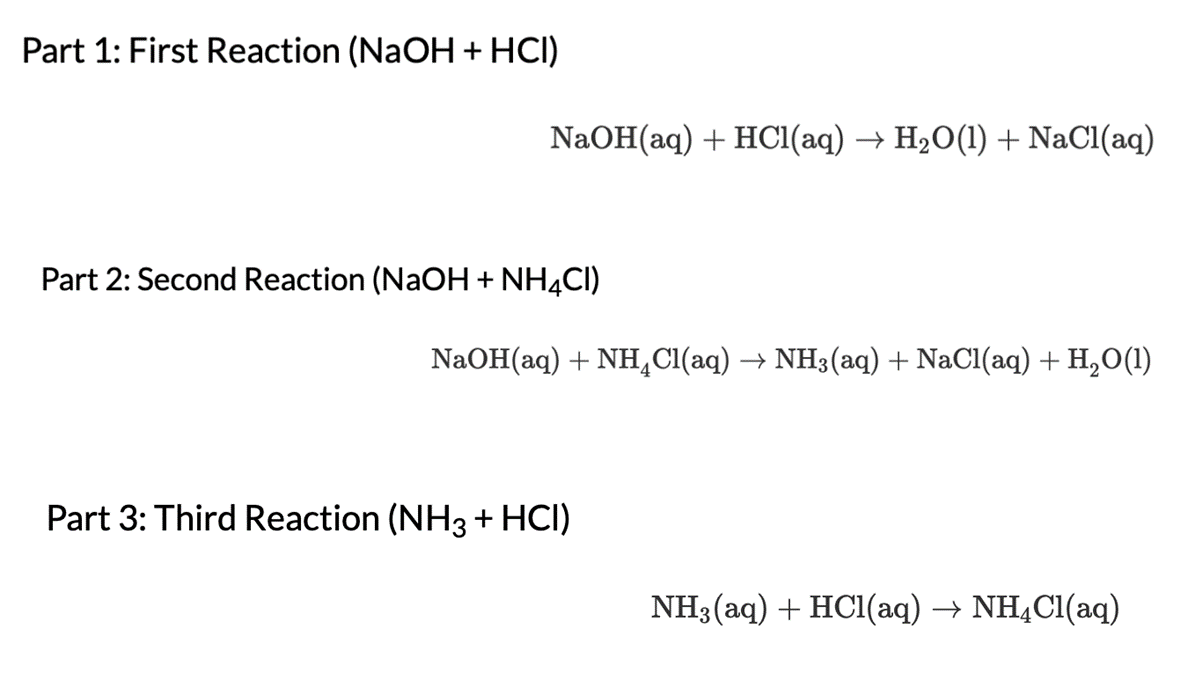
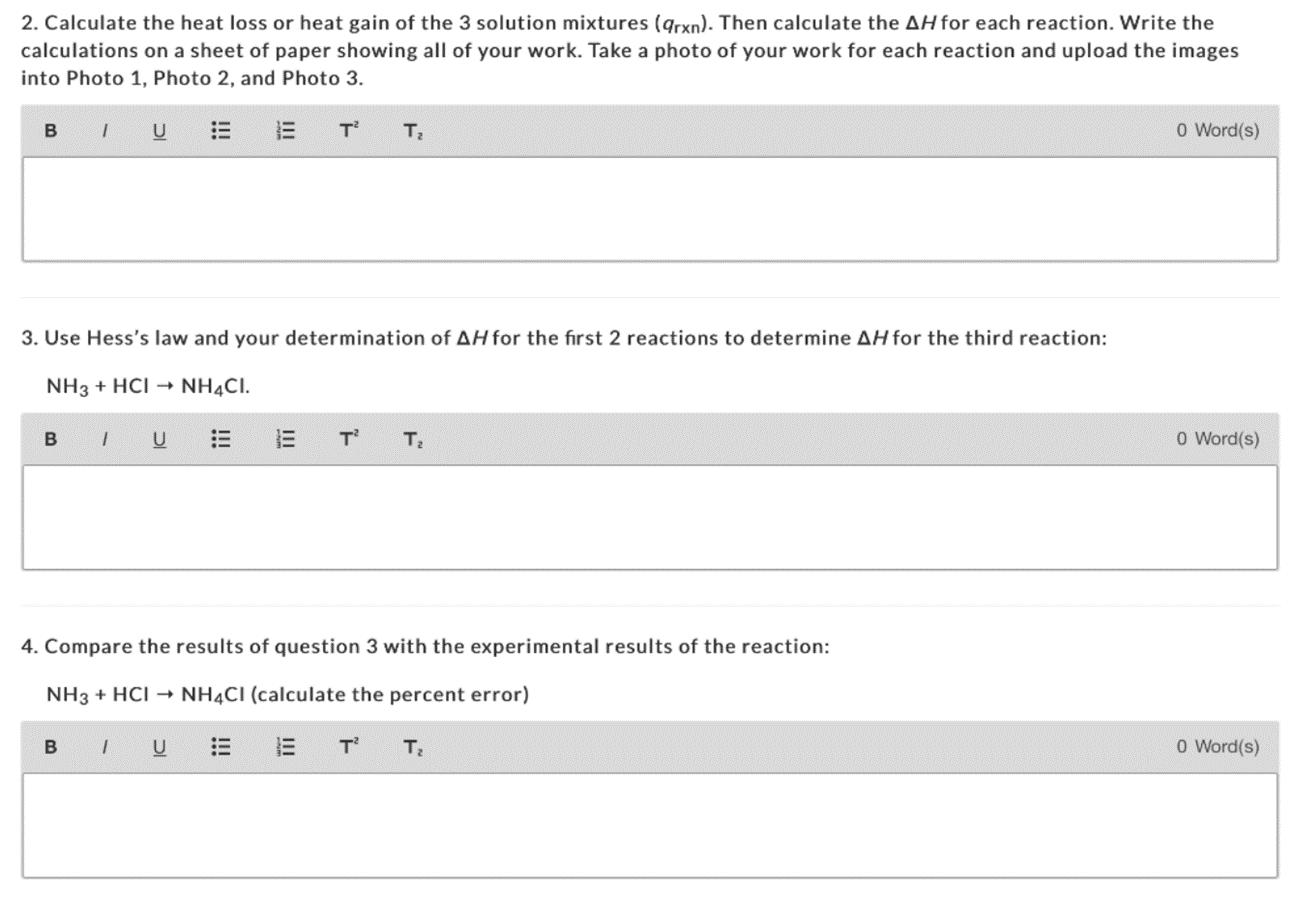
Part 1: First Reaction (NaOH + HCI) NaOH(aq) + HCl(aq) HO(1) + NaCl(aq) Part 2: Second Reaction (NaOH + NH4CI) NaOH(aq) + NHCl(aq) NH(aq) + NaCl(aq) + HO(1) Part 3: Third Reaction (NH3 + HCI) NH3(aq) + HCl(aq) NH4Cl(aq) 2. Calculate the heat loss or heat gain of the 3 solution mixtures (arxn). Then calculate the AH for each reaction. Write the calculations on a sheet of paper showing all of your work. Take a photo of your work for each reaction and upload the images into Photo 1, Photo 2, and Photo 3. B IU B 1 U 3. Use Hess's law and your determination of AH for the first 2 reactions to determine AH for the third reaction: NH3 + HCI NH4CI. : T E E T T T 4. Compare the results of question 3 with the experimental results of the reaction: NH3 + HCI NH4CI (calculate the percent error) BIU T T 0 Word(s) O Word(s) 0 Word(s)
Step by Step Solution
★★★★★
3.47 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Heat of 2xn Reaction 1 E of reaction Qran 4H Helle rxn SH 2xn AH NaOH aq Hce ag Reaction 2 Na...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


