Answered step by step
Verified Expert Solution
Question
1 Approved Answer
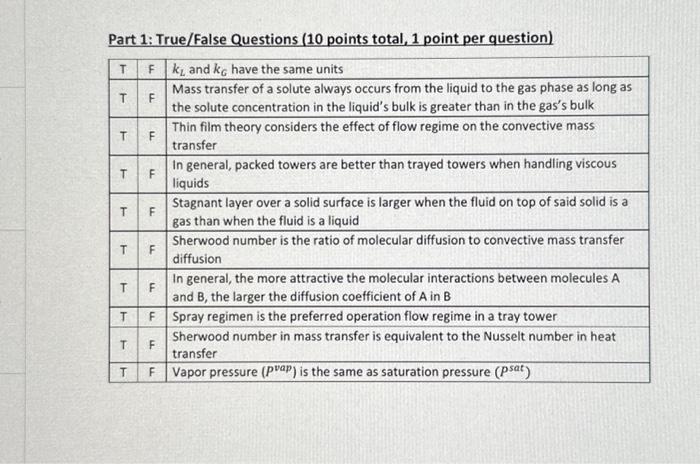
Part 1: True/False Questions (10 points total, 1 point per question) T k and k have the same units T Mass transfer of a solute
Part 1: True/False Questions (10 points total, 1 point per question) T k and k have the same units T Mass transfer of a solute always occurs from the liquid to the gas phase as long as the solute concentration in the liquid's bulk is greater than in the gas's bulk Thin film theory considers the effect of flow regime on the convective mass transfer T T T T T T T T F F LL F LL F F F LL LL F LL F LL In general, packed towers are better than trayed towers when handling viscous liquids Stagnant layer over a solid surface is larger when the fluid on top of said solid is a gas than when the fluid is a liquid Sherwood number is the ratio of molecular diffusion to convective mass transfer diffusion In general, the more attractive the molecular interactions between molecules A and B, the larger the diffusion coefficient of A in B Spray regimen is the preferred operation flow regime in a tray tower Sherwood number in mass transfer is equivalent to the Nusselt number in heat transfer Vapor pressure (pap) is the same as saturation pressure (psat)

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


