Question
Part 2: Clausius-Clapeyron Equation (Vapor pressure, temperature, and Delta H ) Substances that vaporize easily are volatile (weaker IMF) and those that take more energy
Part 2: Clausius-Clapeyron Equation (Vapor pressure, temperature, and
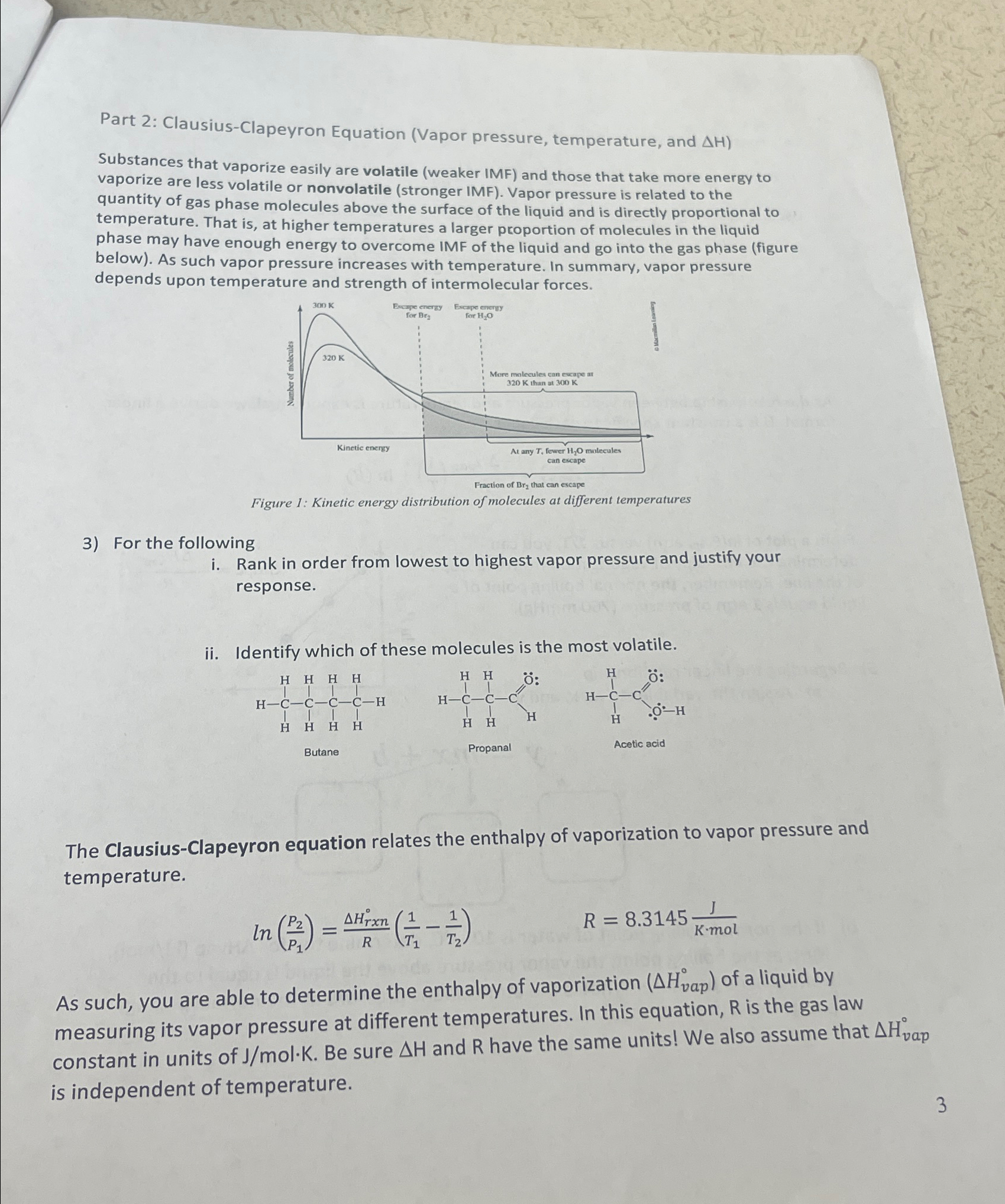
\\\\Delta H)\ Substances that vaporize easily are volatile (weaker IMF) and those that take more energy to vaporize are less volatile or nonvolatile (stronger IMF). Vapor pressure is related to the quantity of gas phase molecules above the surface of the liquid and is directly proportional to temperature. That is, at higher temperatures a larger proportion of molecules in the liquid phase may have enough energy to overcome IMF of the liquid and go into the gas phase (figure below). As such vapor pressure increases with temperature. In summary, vapor pressure depends upon temperature and strength of intermolecular forces.\ Figure 1: Kinetic energy aistribution of motecutes at rem temperuites\ For the following\ i. Rank in order from lowest to highest vapor pressure and justify your response.\ The Clausius-Clapeyron equation relates the enthalpy of vaporization to vapor pressure and temperature.\
ln((P_(2))/(P_(1)))=(\\\\Delta H_(rxn)\\\\deg )/(R)((1)/(T_(1))-(1)/(T_(2)))\
R=8.3145(J)/(K*mol)\ As such, you are able to determine the enthalpy of vaporization (
\\\\Delta H_(vap)\\\\deg ) of a liquid by measuring its vapor pressure at different temperatures. In this equation,
Ris the gas law constant in units of
(J)/(m)ol*K. Be sure
\\\\Delta Hand
Rhave the same units! We also assume that
\\\\Delta H_(vap)\\\\deg is independent of temperature.\ 3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


