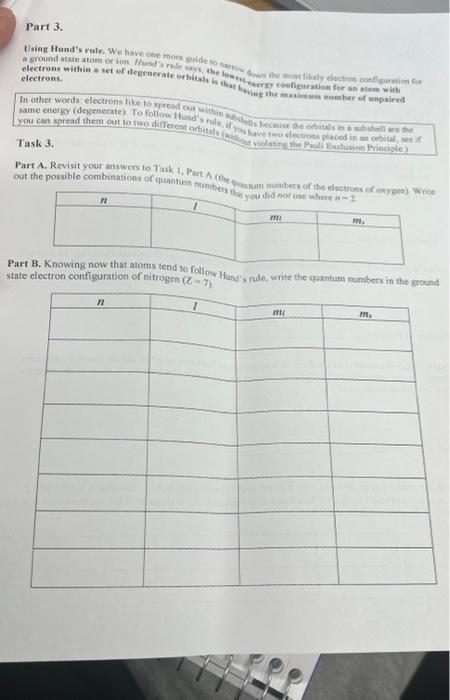
Part 3. Using Hund's rule. We bave one thoce guide to nuphow down the mont likely electron con fipuration for: the thing the makimenas number of enpaired Task 3. Part A. Revisit your answer to Task 1, Part A (the 6. out the possible combinations of quantumin tauna Part B. Nnowng now mar anoms tend to followv Hond's rule, write the quantum nambers in the ground state electron configaration of nitrogen (Z7). Part 4. Patting it all toeether. The periodic sable provides a quick way to detcmine an atom's valence (outer) For non-metal elements, the valence elecirons vere the ones fourd in the x and p orbitals of the outermont shell. Task 4. Using the periodic table at the end of this activity and the itilormation provided, answer the following. questions Part A. Review Task 3. Part B. How many valence elections does nitrogen have? Number of electrons: Part B. Elements in the same group (column) have the szme nuanber of valence clectrons. How many valence electrons does phosphorus have? Number of electrons: Part C. Since the number of electrons in a neutral atom increases with the atomic number, adjacent: elements in the same row should differ in the number of valence elactrons by 1 How many valence electrons does silicon have? Number of electrons: Part D. Knowing that an element with a filled shell is psticularly stable, how many total s and p electrons do you expect to find in the most stable elements? Write down at least six elements that y expect will have this special stability. Part 3. Using Hund's rule. We bave one thoce guide to nuphow down the mont likely electron con fipuration for: the thing the makimenas number of enpaired Task 3. Part A. Revisit your answer to Task 1, Part A (the 6. out the possible combinations of quantumin tauna Part B. Nnowng now mar anoms tend to followv Hond's rule, write the quantum nambers in the ground state electron configaration of nitrogen (Z7). Part 4. Patting it all toeether. The periodic sable provides a quick way to detcmine an atom's valence (outer) For non-metal elements, the valence elecirons vere the ones fourd in the x and p orbitals of the outermont shell. Task 4. Using the periodic table at the end of this activity and the itilormation provided, answer the following. questions Part A. Review Task 3. Part B. How many valence elections does nitrogen have? Number of electrons: Part B. Elements in the same group (column) have the szme nuanber of valence clectrons. How many valence electrons does phosphorus have? Number of electrons: Part C. Since the number of electrons in a neutral atom increases with the atomic number, adjacent: elements in the same row should differ in the number of valence elactrons by 1 How many valence electrons does silicon have? Number of electrons: Part D. Knowing that an element with a filled shell is psticularly stable, how many total s and p electrons do you expect to find in the most stable elements? Write down at least six elements that y expect will have this special stability