Answered step by step
Verified Expert Solution
Question
1 Approved Answer
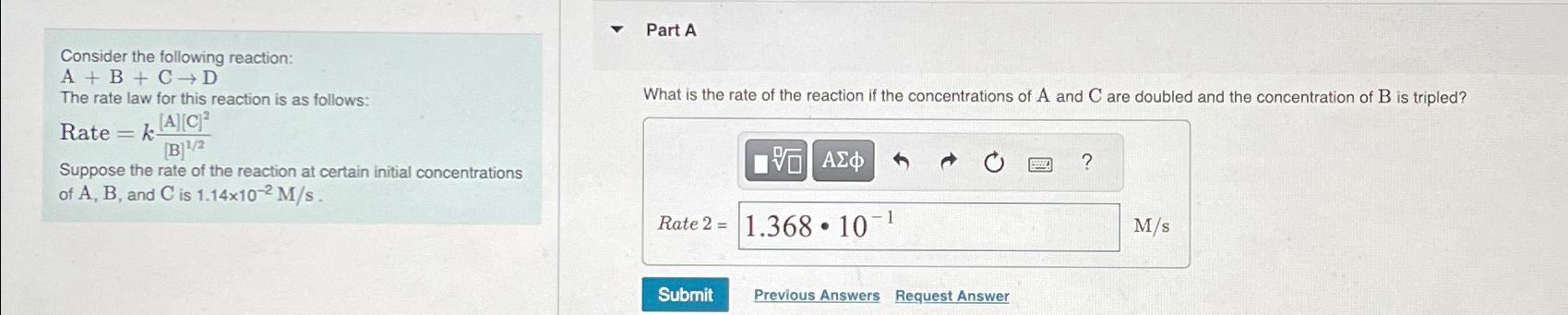
Part A Consider the following reaction: A+B+C->D The rate law for this reaction is as follows: Rate =k([A][C]^(2))/([(B)]^((1)/(2))) Suppose the rate of the
Part A\ Consider the following reaction:\
A+B+C->D\ The rate law for this reaction is as follows:\ Rate
=k([A][C]^(2))/([(B)]^((1)/(2)))\ Suppose the rate of the reaction at certain initial concentrations of
A,B, and
Cis
1.14\\\\times 10^(-2)(M)/(s).\ What is the rate of the reaction if the concentrations of
Aand
Care doubled and the concentration of
Bis tripled?\ Rate
2=\
(M)/(s)\ Previous Answers\ Request Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


