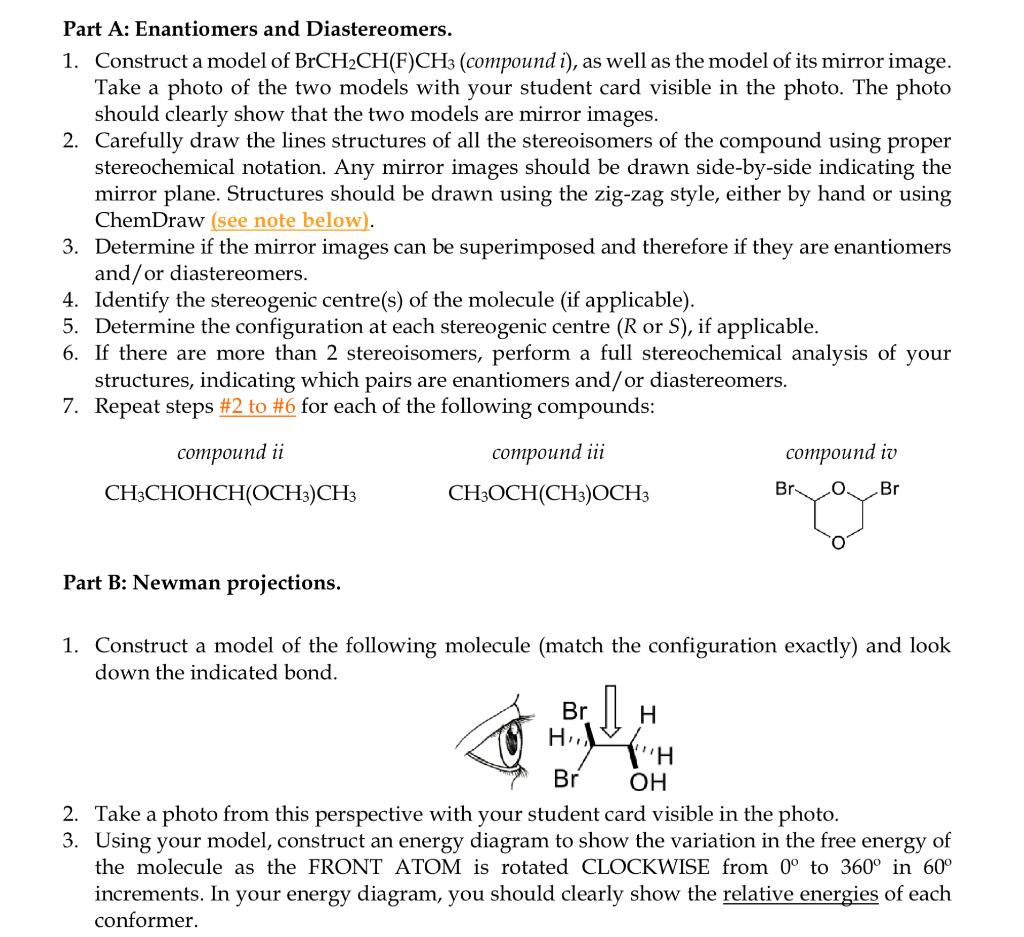
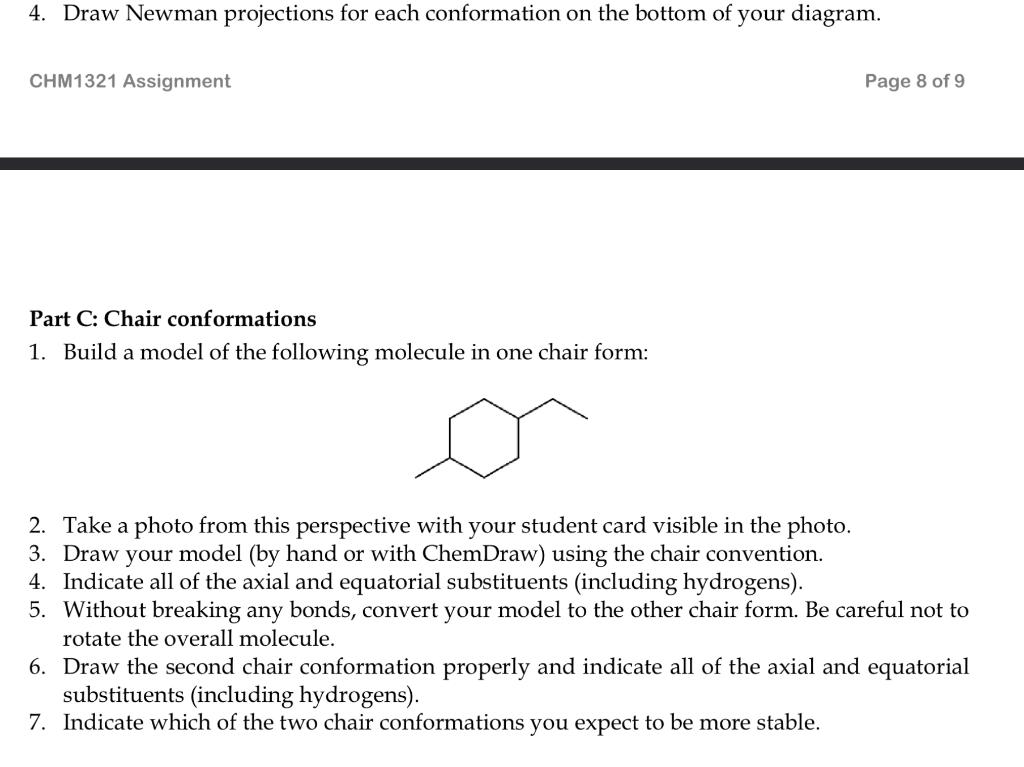
Part A: Enantiomers and Diastereomers. 1. Construct a model of BrCH2CH(F)CH3 (compound i ), as well as the model of its mirror image. Take a photo of the two models with your student card visible in the photo. The photo should clearly show that the two models are mirror images. 2. Carefully draw the lines structures of all the stereoisomers of the compound using proper stereochemical notation. Any mirror images should be drawn side-by-side indicating the mirror plane. Structures should be drawn using the zig-zag style, either by hand or using ChemDraw (see note below). 3. Determine if the mirror images can be superimposed and therefore if they are enantiomers and/or diastereomers. 4. Identify the stereogenic centre(s) of the molecule (if applicable). 5. Determine the configuration at each stereogenic centre (R or S), if applicable. 6. If there are more than 2 stereoisomers, perform a full stereochemical analysis of your structures, indicating which pairs are enantiomers and/or diastereomers. 7. Repeat steps \#2 to \#6 for each of the following compounds: Part B: Newman projections. 1. Construct a model of the following molecule (match the configuration exactly) and look down the indicated bond. 2. Take a photo from this perspective with your student card visible in the photo. 3. Using your model, construct an energy diagram to show the variation in the free energy of the molecule as the FRONT ATOM is rotated CLOCKWISE from 0 to 360 in 60 increments. In your energy diagram, you should clearly show the relative energies of each conformer. Part C: Chair conformations 1. Build a model of the following molecule in one chair form: 2. Take a photo from this perspective with your student card visible in the photo. 3. Draw your model (by hand or with ChemDraw) using the chair convention. 4. Indicate all of the axial and equatorial substituents (including hydrogens). 5. Without breaking any bonds, convert your model to the other chair form. Be careful not to rotate the overall molecule. 6. Draw the second chair conformation properly and indicate all of the axial and equatorial substituents (including hydrogens). 7. Indicate which of the two chair conformations you expect to be more stable. Part A: Enantiomers and Diastereomers. 1. Construct a model of BrCH2CH(F)CH3 (compound i ), as well as the model of its mirror image. Take a photo of the two models with your student card visible in the photo. The photo should clearly show that the two models are mirror images. 2. Carefully draw the lines structures of all the stereoisomers of the compound using proper stereochemical notation. Any mirror images should be drawn side-by-side indicating the mirror plane. Structures should be drawn using the zig-zag style, either by hand or using ChemDraw (see note below). 3. Determine if the mirror images can be superimposed and therefore if they are enantiomers and/or diastereomers. 4. Identify the stereogenic centre(s) of the molecule (if applicable). 5. Determine the configuration at each stereogenic centre (R or S), if applicable. 6. If there are more than 2 stereoisomers, perform a full stereochemical analysis of your structures, indicating which pairs are enantiomers and/or diastereomers. 7. Repeat steps \#2 to \#6 for each of the following compounds: Part B: Newman projections. 1. Construct a model of the following molecule (match the configuration exactly) and look down the indicated bond. 2. Take a photo from this perspective with your student card visible in the photo. 3. Using your model, construct an energy diagram to show the variation in the free energy of the molecule as the FRONT ATOM is rotated CLOCKWISE from 0 to 360 in 60 increments. In your energy diagram, you should clearly show the relative energies of each conformer. Part C: Chair conformations 1. Build a model of the following molecule in one chair form: 2. Take a photo from this perspective with your student card visible in the photo. 3. Draw your model (by hand or with ChemDraw) using the chair convention. 4. Indicate all of the axial and equatorial substituents (including hydrogens). 5. Without breaking any bonds, convert your model to the other chair form. Be careful not to rotate the overall molecule. 6. Draw the second chair conformation properly and indicate all of the axial and equatorial substituents (including hydrogens). 7. Indicate which of the two chair conformations you expect to be more stable








