Question
Part A Estimate the volume, V, of one sodium atom (Na) using its metallic radius of 186 pm. Express your answer with the appropriate
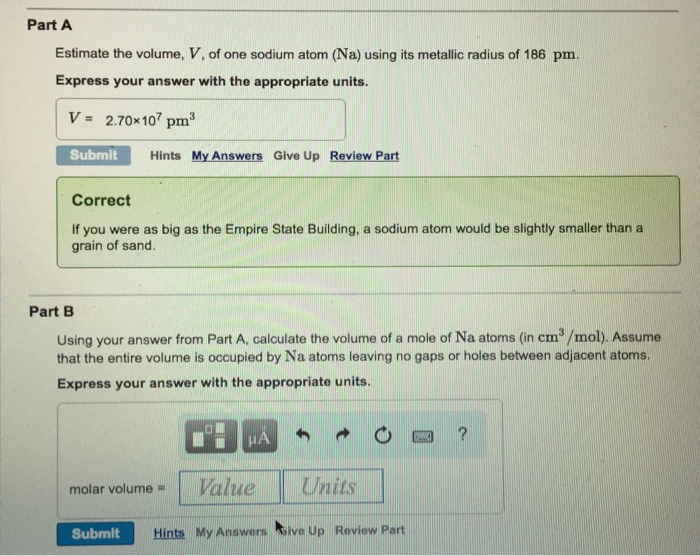
Part A Estimate the volume, V, of one sodium atom (Na) using its metallic radius of 186 pm. Express your answer with the appropriate units. V = 2.70x107 pm Submit Hints My Answers Give Up Review Part Correct If you were as big as the Empire State Building, a sodium atom would be slightly smaller than a grain of sand. Part B Using your answer from Part A, calculate the volume of a mole of Na atoms (in cm/mol). Assume that the entire volume is occupied by Na atoms leaving no gaps or holes between adjacent atoms. Express your answer with the appropriate units. molar volume = HA Value Units Submit Hints My Answers ive Up Review Part DW ?
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Part A The radius of the atom of the sodium atom is 1...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics The Exploration & Analysis Of Data
Authors: Roxy Peck, Jay L. Devore
7th Edition
0840058012, 978-0840058010
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



