Answered step by step
Verified Expert Solution
Question
1 Approved Answer
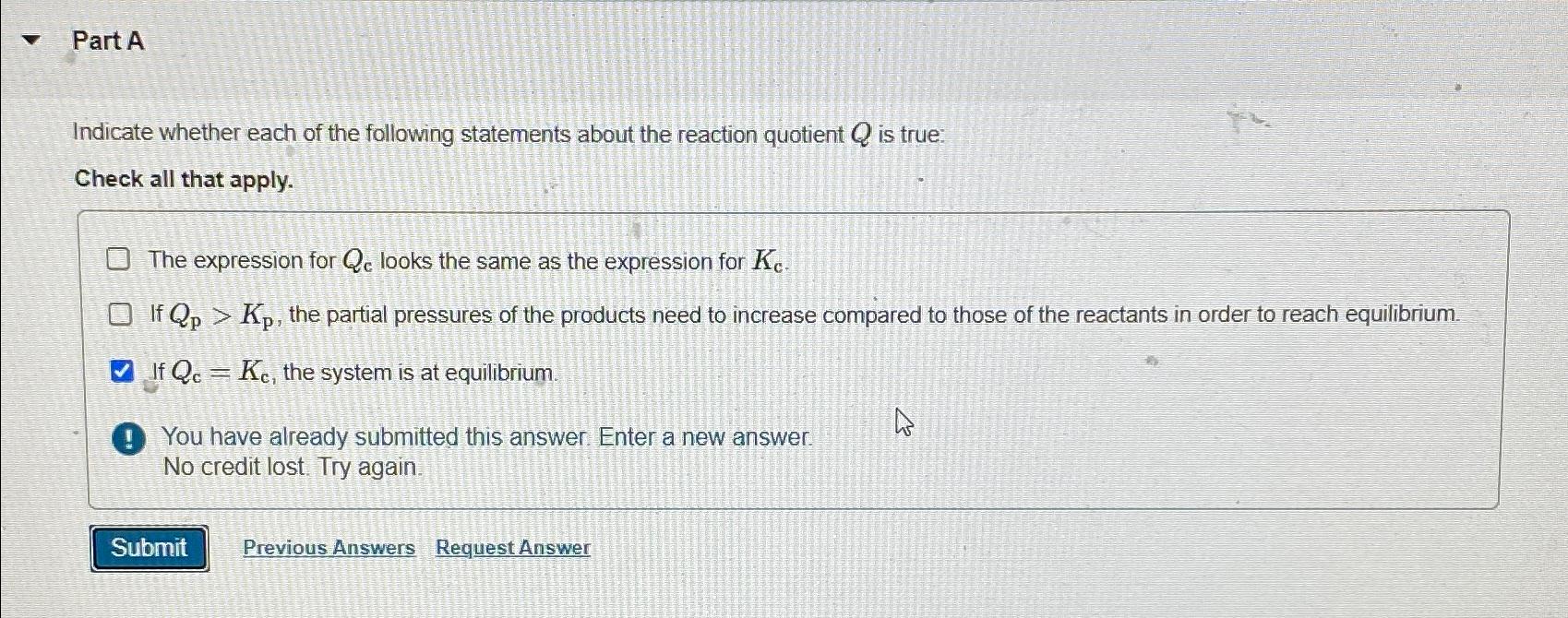
Part A Indicate whether each of the following statements about the reaction quotient Q is true: Check all that apply. The expression for Q c
Part A
Indicate whether each of the following statements about the reaction quotient is true:
Check all that apply.
The expression for looks the same as the expression for
If the partial pressures of the products need to increase compared to those of the reactants in order to reach equilibrium.
If the system is at equilibrium.
You have already submitted this answer. Enter a new answer.
No credit lost. Try again.
Previous Answers
Request Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


