Question
Part A IP A 1.5-kg block of ice is initially at a temperature of -2.0 C. If 2.6105 J of heat are added to
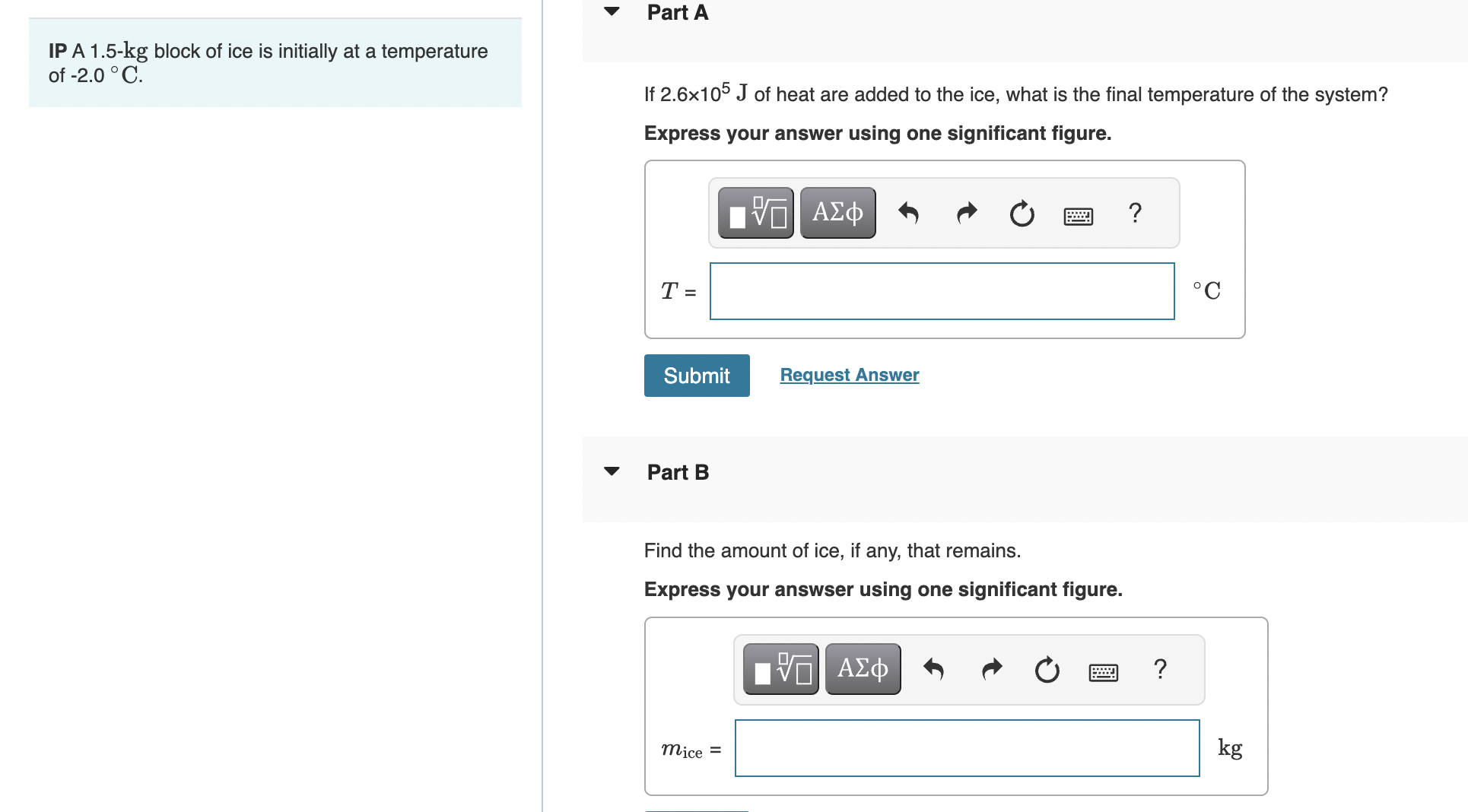
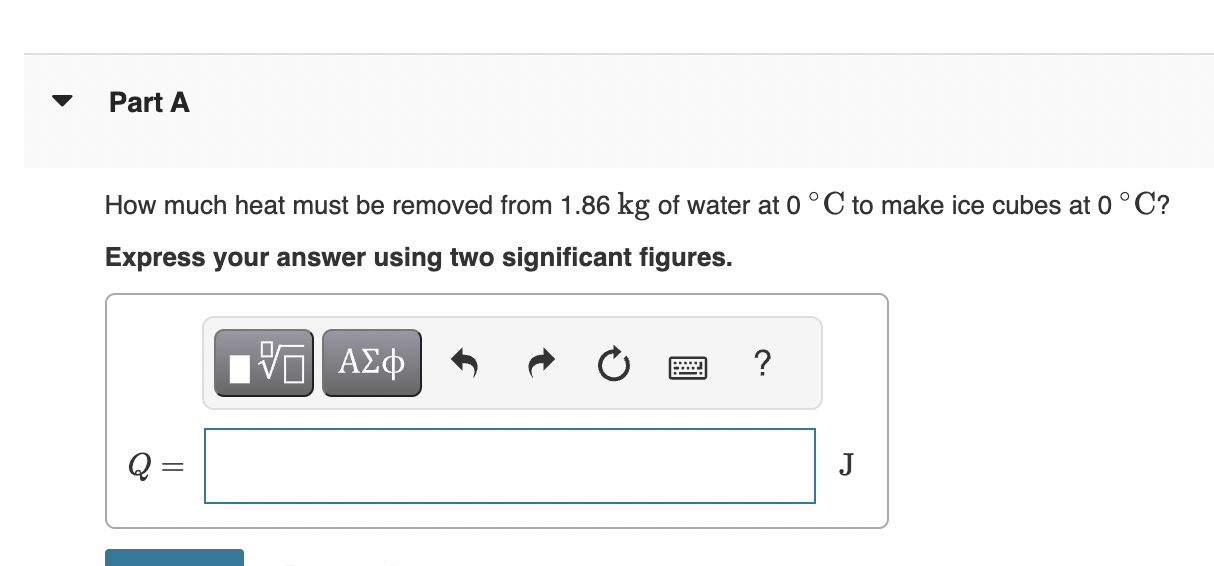
Part A IP A 1.5-kg block of ice is initially at a temperature of -2.0 C. If 2.6105 J of heat are added to the ice, what is the final temperature of the system? Express your answer using one significant figure. VA ? T = Submit Request Answer Part B Find the amount of ice, if any, that remains. Express your answser using one significant figure. mice = ? C kg Part A How much heat must be removed from 1.86 kg of water at 0C to make ice cubes at 0C? Express your answer using two significant figures. 0 VO ? J
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: James S. Walker
5th edition
978-0133498493, 9780321909107, 133498492, 0321909100, 978-0321976444
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



