Answered step by step
Verified Expert Solution
Question
1 Approved Answer
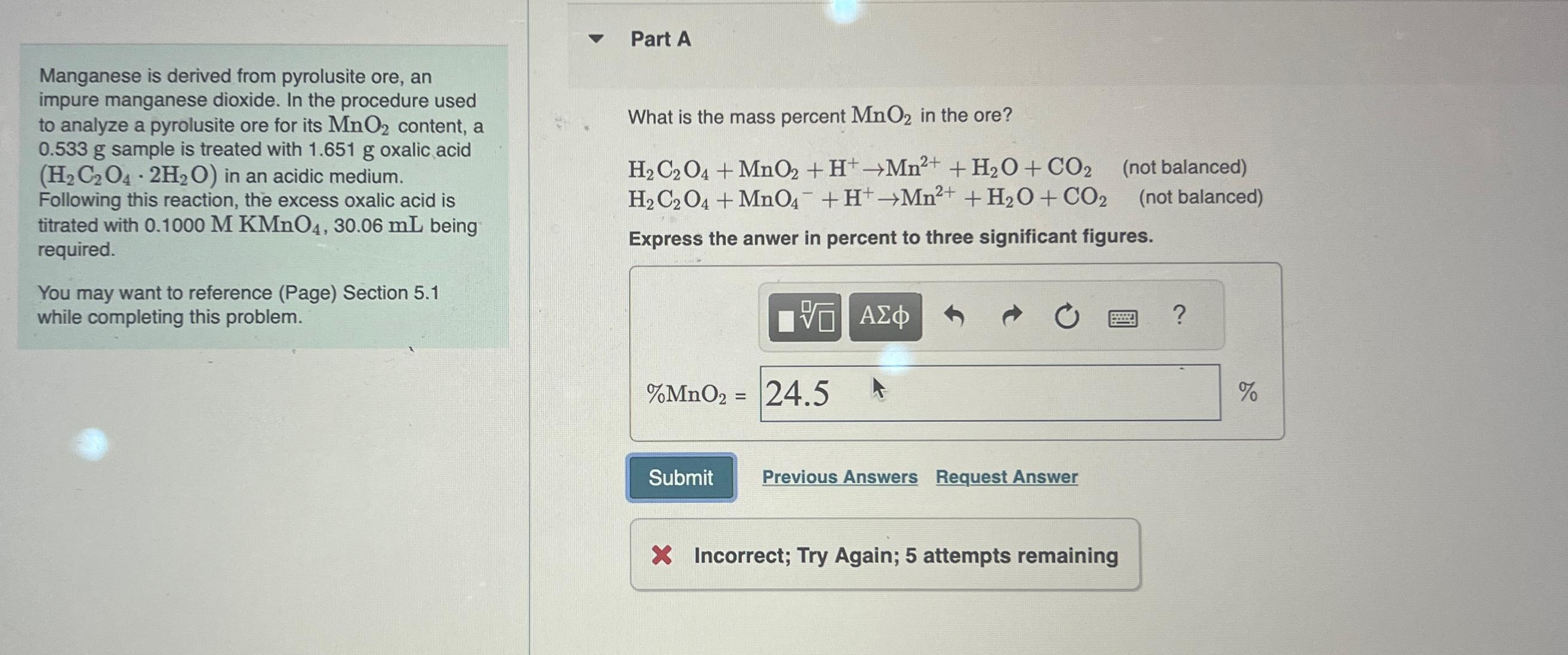
Part A Manganese is derived from pyrolusite ore, an impure manganese dioxide. In the procedure used to analyze a pyrolusite ore for its M n
Part A
Manganese is derived from pyrolusite ore, an impure manganese dioxide. In the procedure used to analyze a pyrolusite ore for its content, a sample is treated with oxalic acid in an acidic medium.
Following this reaction, the excess oxalic acid is titrated with being required.
You may want to reference Page Section while completing this problem.
What is the mass percent in the ore?
balanced
balanced
Express the anwer in percent to three significant figures.
Previous Answers
Request Answer
Incorrect; Try Again; attempts remaining
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


