Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A) Part B) Part C) K = 1.8x10^-5 at 25C Consider the reaction: Calculate the value of the equilibrium constant (Kc). Fe3+(aq)+SCN(aq)FeSCN2+(aq) Express your
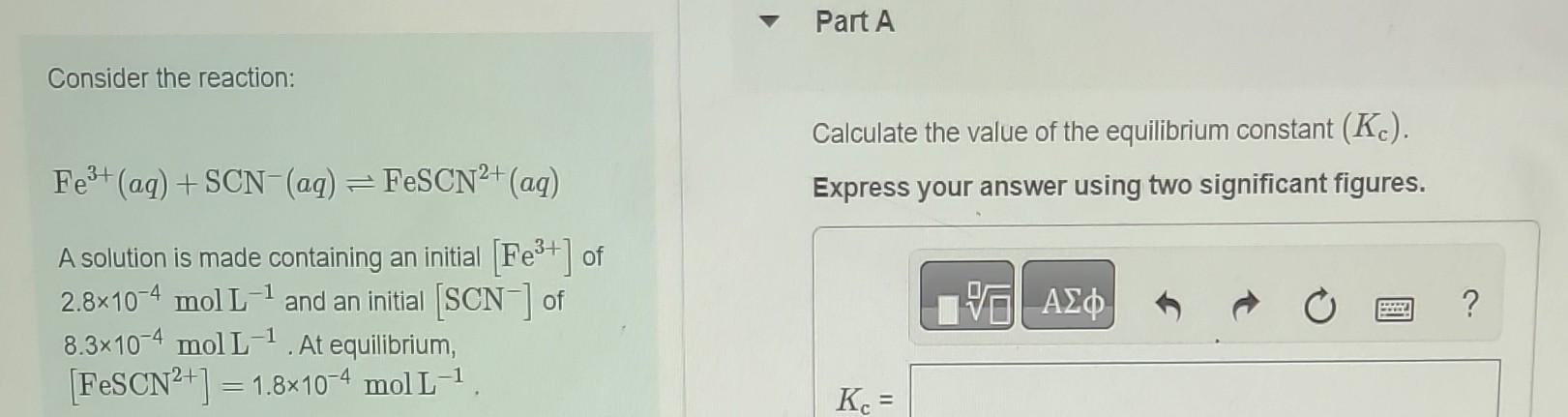
Part A)
Part B)

Part C) K = 1.8x10^-5 at 25C

Consider the reaction: Calculate the value of the equilibrium constant (Kc). Fe3+(aq)+SCN(aq)FeSCN2+(aq) Express your answer using two significant figures. A solution is made containing an initial [Fe3+] of 2.8104molL1 and an initial [SCN]of 8.3104mol1. At equilibrium, [FeSCN2+]=1.8104molL1 Consider the reaction and associated equilibrium Find the equilibrium concentrations of A and B for a=2 and b=2. Assume that the initial concentration of A is constant: 1.0molL1 and that no B is present at the beginning of the reaction. aA(g)bB(g),K=2.5 Express your answers using two significant figures separated by a comma. If a solution initially contains 0.265molL1CH3COOH, what is the equilibrium concentration of H3O+; 25C? Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


