Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A PC13 Br2 is a nonpolar molecule. Based on this information, determine the Cl-P-Cl bond angle, the Br-P-Br bond angle, and the Cl-P-Br
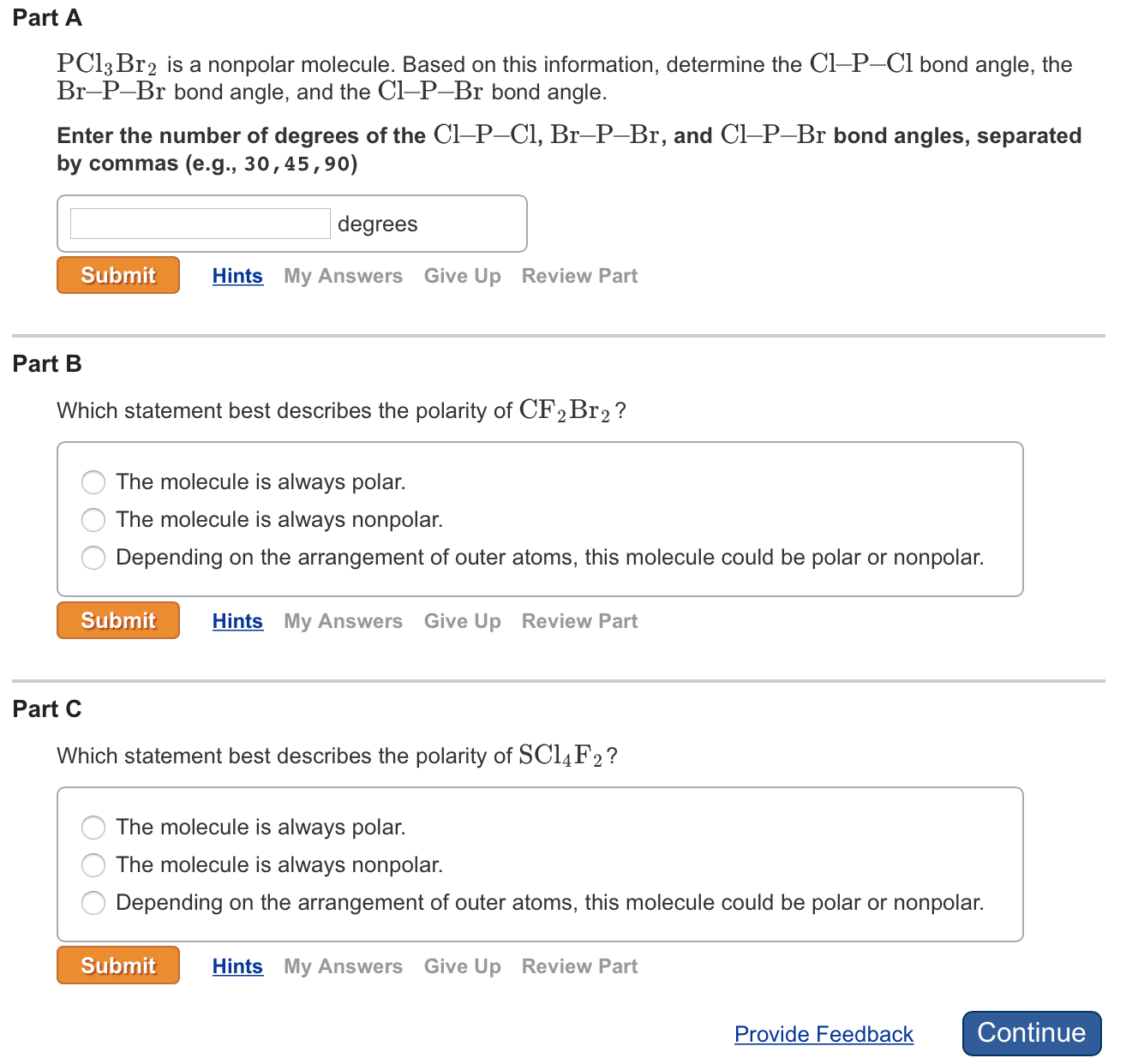
Part A PC13 Br2 is a nonpolar molecule. Based on this information, determine the Cl-P-Cl bond angle, the Br-P-Br bond angle, and the Cl-P-Br bond angle. Enter the number of degrees of the Cl-P-Cl, Br-P-Br, and Cl-P-Br bond angles, separated by commas (e.g., 30, 45,90) degrees Submit Hints My Answers Give Up Review Part Part B Which statement best describes the polarity of CFBr ? The molecule is always polar. The molecule is always nonpolar. Depending on the arrangement of outer atoms, this molecule could be polar or nonpolar. Submit Hints My Answers Give Up Review Part Part C Which statement best describes the polarity of SC14 F? The molecule is always polar. The molecule is always nonpolar. Depending on the arrangement of outer atoms, this molecule could be polar or nonpolar. Submit Hints My Answers Give Up Review Part Provide Feedback Continue
Step by Step Solution
★★★★★
3.59 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Part A Answer 12018090 Explanation P has five electrons in its outermost shell and bonded to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


