Answered step by step
Verified Expert Solution
Question
1 Approved Answer
0.50 g of sodium hydroxide (NaOH) pellets are dissolved in water to make 7.5 L of solution. What is the pH of this solution'
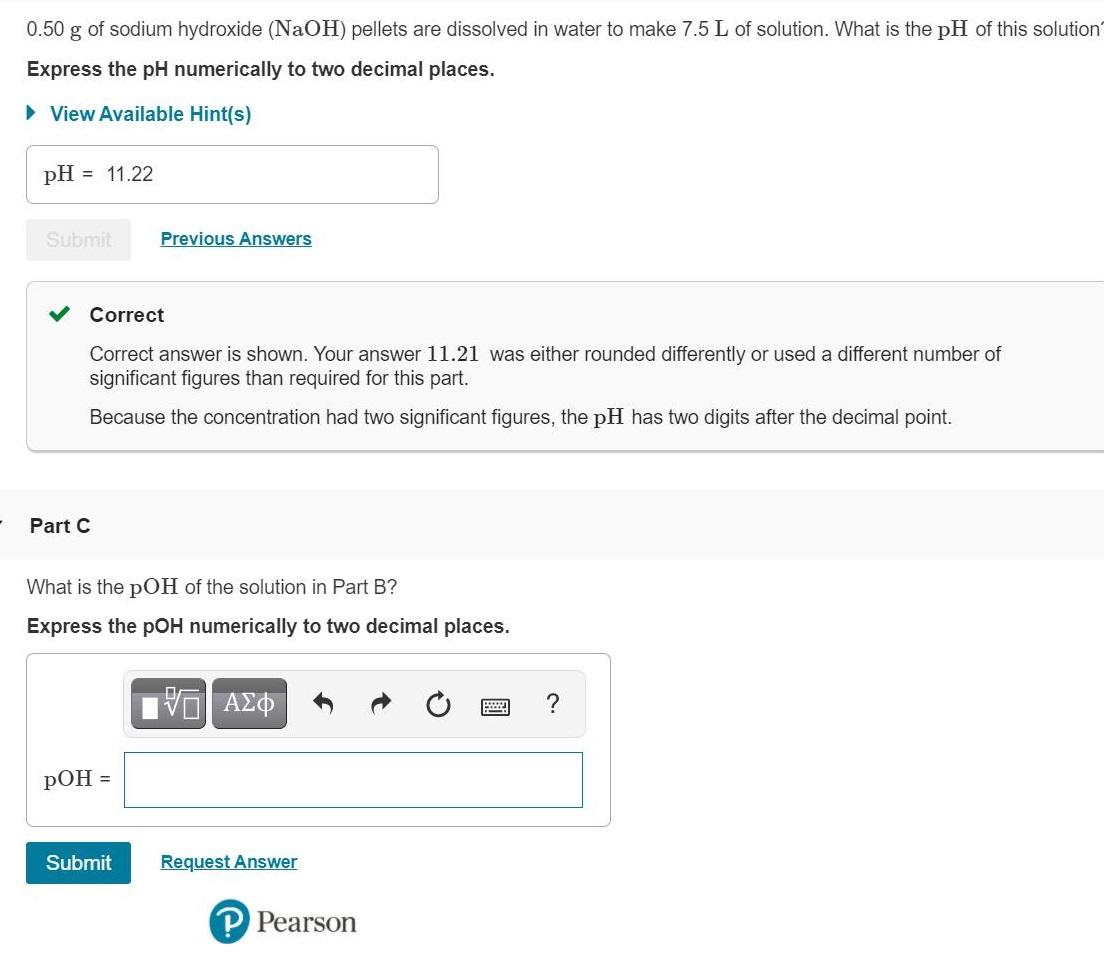
0.50 g of sodium hydroxide (NaOH) pellets are dissolved in water to make 7.5 L of solution. What is the pH of this solution' Express the pH numerically to two decimal places. View Available Hint(s) pH = 11.22 Submit Previous Answers Correct Correct answer is shown. Your answer 11.21 was either rounded differently or used a different number of significant figures than required for this part. Because the concentration had two significant figures, the pH has two digits after the decimal point. Part C What is the pOH of the solution in Part B? Express the pOH numerically to two decimal places. V pOH = Submit Request Answer P Pearson
Step by Step Solution
★★★★★
3.32 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Given Mass 5 NgO H 0 509 vol of solution 75L To find pOH Formu...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


