Question
A solution of water (Kf point of pure water is 0.00 C. = 1.86 C/m) and glucose freezes at 1.75C. What is the molal
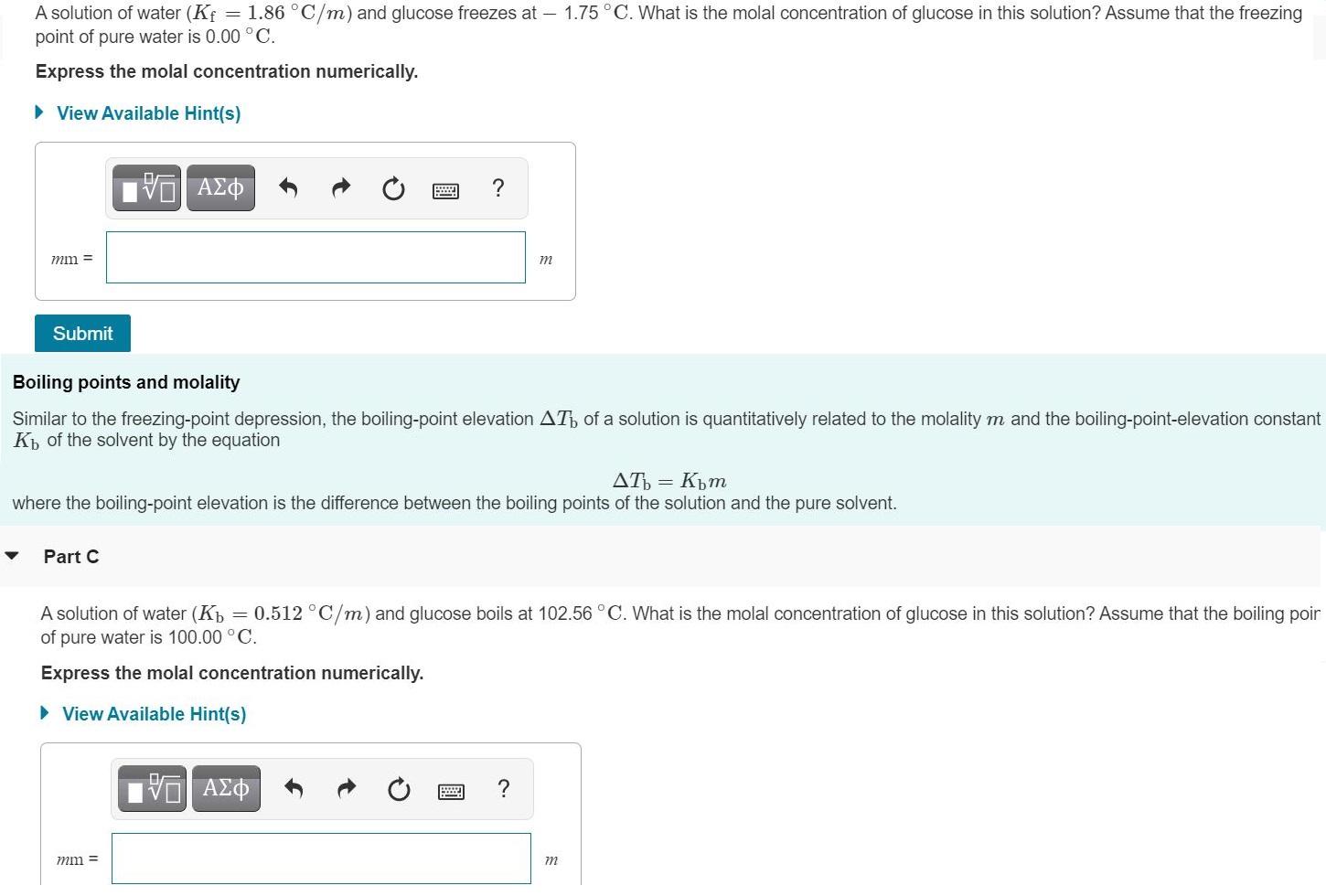
A solution of water (Kf point of pure water is 0.00 C. = 1.86 C/m) and glucose freezes at 1.75C. What is the molal concentration of glucose in this solution? Assume that the freezing Express the molal concentration numerically. View Available Hint(s) mm = m Submit Boiling points and molality Similar to the freezing-point depression, the boiling-point elevation AT of a solution is quantitatively related to the molality m and the boiling-point-elevation constant K of the solvent by the equation AT = Kpm where the boiling-point elevation is the difference between the boiling points of the solution and the pure solvent. Part C A solution of water (Kp = 0.512 C/m) and glucose boils at 102.56C. What is the molal concentration of glucose in this solution? Assume that the boiling poir of pure water is 100.00 C. Express the molal concentration numerically. View Available Hint(s) mm = m
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Practical Management Science
Authors: Wayne L. Winston, Christian Albright
5th Edition
1305631540, 1305631544, 1305250907, 978-1305250901
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



