Question
Part B: Calculate the change in internal energy ?U ofthe gas as it expands isothermally from V0 to the finalvolume fvV0. Here, fv is the
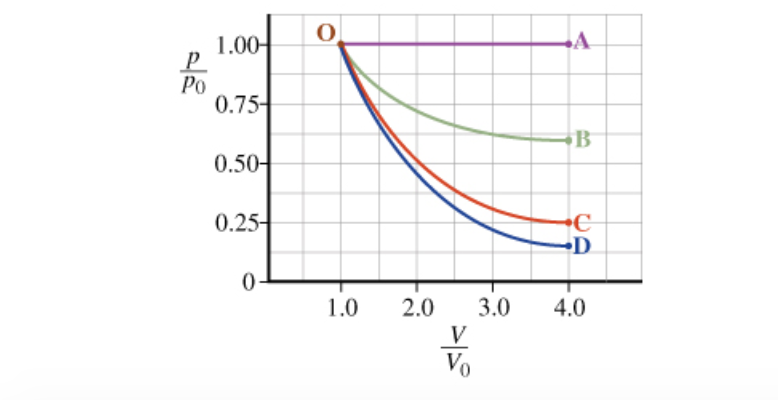
Part B: Calculate the change in internal energy ?U ofthe gas as it expands isothermally from V0 to the finalvolume fvV0. Here, fv is the ratio of final volumeto initial volume; fv=4.0 in this problem.
Express your answer in terms of given quantities.
?U =
Part C: The pressure, temperature and volume of a system changedifferently during the process of isothermal expansion. Indicatewhether each quantity increases (I), remains constant (C), ordecreases (D).
Your answers should describe the changes of pressure,temperature, and volume (in that order). Separate your answers withcommas (e.g., I,C,D means pressure increases, temperatureremains constant, and volume decreases).
pressure, temperature, volume =
Part D: What is the work W done by the gas as itexpands isothermally from V0 to fvV0?
Express the work done in terms of p0, V0, and fv.Use ln for the natural logarithm.
W=
Part E: Calculate the quantity of heat Q that must bedelivered to the gas as it expandsfrom V0 to fvV0.
Express your answer in terms of p0, V0, and fv.Use ln for the natural logarithm.
Q=
Part F: Which of the following statements are true?
- Heat is converted completely into workduring isothermal expansion.
- Isothermal expansion is reversible under ideal conditions.
- During the process of isothermal expansion, the gas does morework than during an isobaric expansion (at constant pressure)between the same initial and final volumes.
Po 1.00 0.75+ 0.50 0.25 0 O 1.0 2.0 V Vo 3.0 A B C D 4.0
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
in c e In isothermal process temp is const So Av0 pressure decr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


