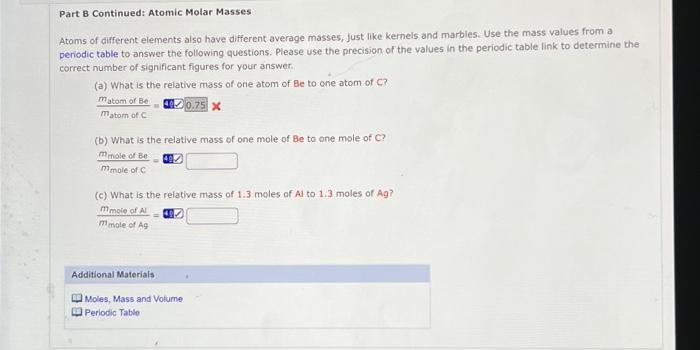
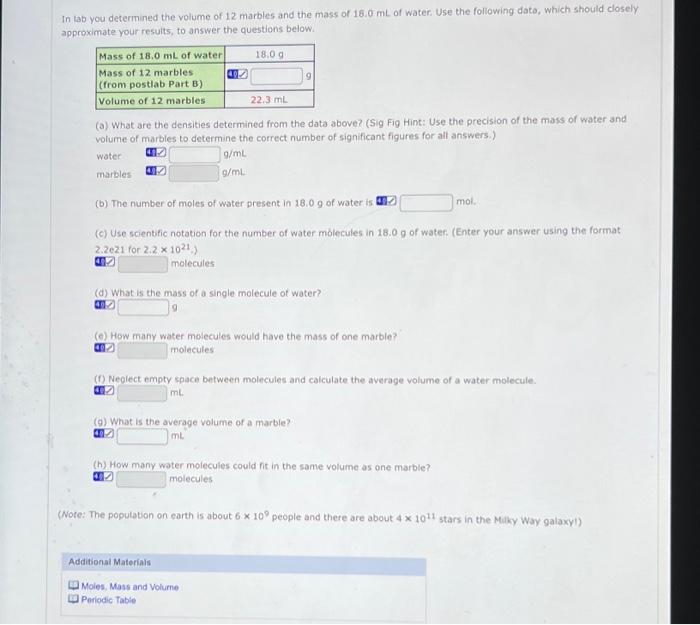
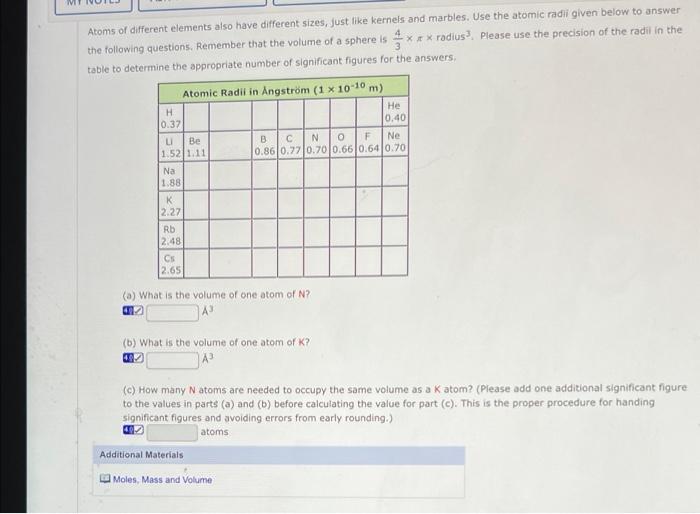
Part B Continued: Atomic Molar Masses Atoms of different elements also have different average masses, just like kernels and marbles. Use the mass values from a periodic table to answer the following questions. Please use the precision of the values in the periodic table link to determine the correct number of significant figures for your answer (a) What is the relative mass of one atom of Be to one atom of C? matom of Be 0.75 x matom ofc (b) What is the relative mass of one mole of Be to one mole of C? mmole of Be mmole of (c) What is the relative mass of 1.3 moles of Al to 1.3 moles of Ag? mmole of A mmole of Ag Additional Materials Moles, Mass and Volume Periodic Table 9 In tob you determined the volume of 12 marbles and the mass of 18.0 mL of water Use the following dato, which should closely approximate your results, to answer the questions below. Mass of 18.0 mL of water 18.09 Mass of 12 marbles (from postlab Part B) Volume of 12 marbles 22.3 mL (a) What are the densities determined from the data above? (Sig Fig Hint: Use the precision of the mass of water and volume of marbles to determine the correct number of significant figures for all answers.) water g/ml marbles g/ml mol (b) The number of moles of water present in 18.0 9 of water is (c) Use scientific notation for the number of water molecules in 18.0 9 of water. (Enter your answer using the format 2.2021 for 2.2 x 1021) molecules (a) What is the mass of a single molecule of water? (How many water molecules would have the mass of one marble? molecules (Neglect empty space between molecules and calculate the average volume of a water molecule. mL (O) What is the average volume or a marble? mu (h) How many water molecules could it in the same volume as one marble? molecules (Note: The population on earth is about 6 * 10 people and there are about 4 * 1011 stars in the Milky Way galaxy!) Additional Materials Moles. Mass and Volume Periodic Table Atoms of different elements also have different sizes, just like kernels and marbles. Use the atomic radii given below to answer the following questions. Remember that the volume of a sphere is *** radius. Please use the precision of the radil in the table to determine the appropriate number of significant figures for the answers, Atomic Radil in Angstrom (1 x 10-10 m) H He 0.37 0.40 u Be B C N O F Ne 1.521.11 0.86 0.27 0.70 0.660.64 0.70 Na 1.88 K 2.27 Rb 2.48 Cs 2.65 () What is the volume of one atom of N? (b) What is the volume of one atom of K? (C) How many Natoms are needed to occupy the same volume as a k atom? (Please add one additional significant figure to the values in parts (a) and (b) before calculating the value for part (c). This is the proper procedure for handing significant figures and avoiding errors from early rounding.) atoms Additional Materials Moles, Mass and Volume