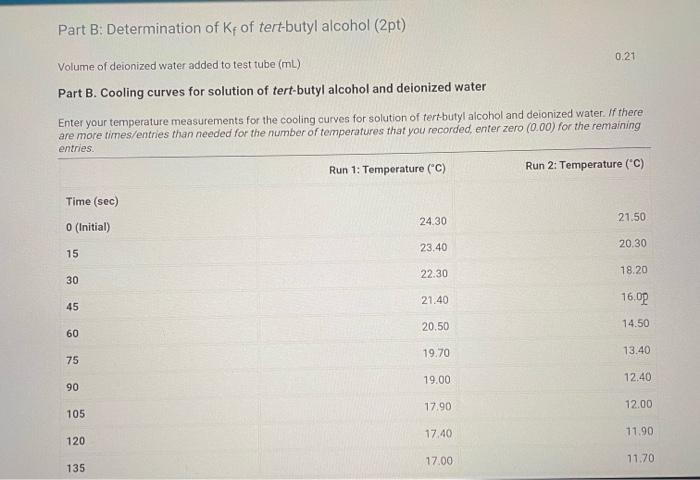
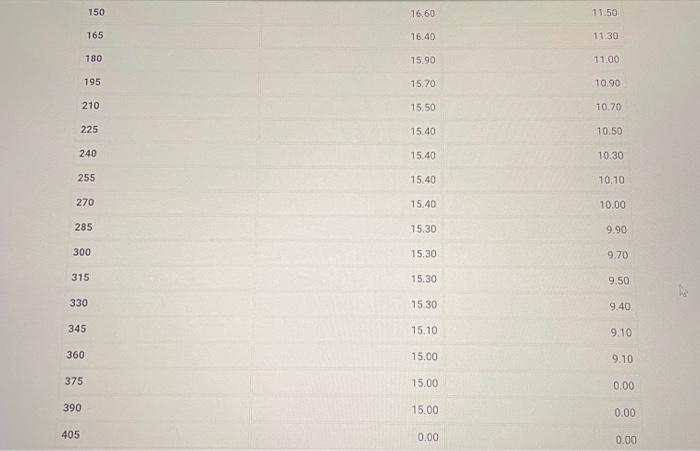
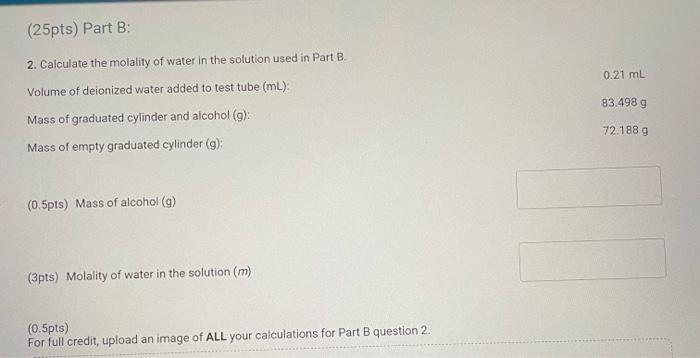
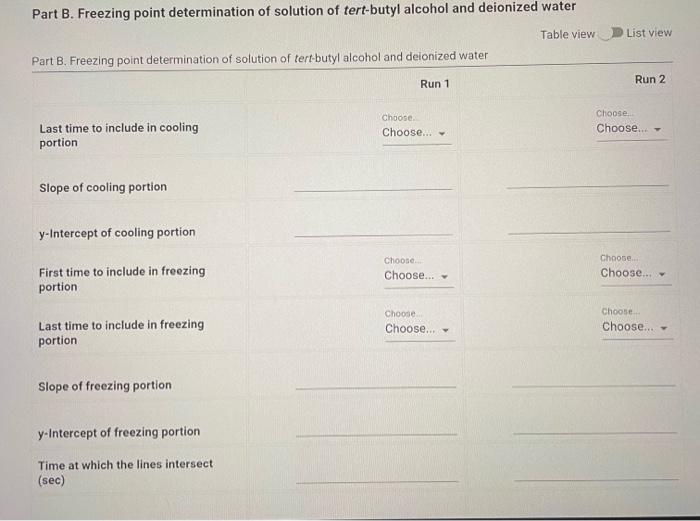
Part B: Determination of Kf of tert-butyl alcohol (2pt) Volume of deionized water added to test tube (mL) Part B. Cooling curves for solution of tert-butyl alcohol and deionized water Enter your temperature measurements for the cooling curves for solution of tertbutyl alcohol and deionized water. If there are more times/entries than needed for the number of temperatures that you recorded, enter zero (0.00) for the remaining (25pts) Part B: 2. Calculate the molality of water in the solution used in Part B. Volume of deionized water added to test tube (mL) : Mass of graduated cylinder and alcohol (g) : Mass of empty graduated cylinder (g) : (0.5pts) Mass of alcohol (g) (3pts) Molality of water in the solution (m) (0.5pts) For full credit, upload an image of ALL your calculations for Part B question 2. Part B. Freezing point determination of solution of tert-butyl alcohol and deionized water Table view List view (1pts) Average freezing point for the solution of teri-butyl alcohol and deionized water ( C ) (2pts) Upload your graphs for Part B here. Make sure you have an appropriate titles, axis labels, and that the equations for the trendlines are displayed on the chart. You can upload your graphs directly (as a .Xlsx, csv, or .txt file) or upload a screen shot or photo of your graphs (as a .pdi, .jpg. .png, or heic file). Note: Hand drawn araphs are not accepted. (1pts) For full credit, upload an image of ALL your calculations for Part B question 3. 4. Calculate T for the solution in Part B. Remember T=TpureTsolution:. (3pts) T(C) (1pts) For full credit, upload an image of your calculation for Part B question 4. Browse your files to upload or Drag and Drop Max attachments: 5 I Max Size: 20.00MM each 5. Calculate the freezing point depression constant, Kf, for tert-butyl alcohol using the T calculated in question 4. (Apts) Kf(C/m) (1pts) For full credit, upload an image of your calculation for Part B question 5. Part B: Determination of Kf of tert-butyl alcohol (2pt) Volume of deionized water added to test tube (mL) Part B. Cooling curves for solution of tert-butyl alcohol and deionized water Enter your temperature measurements for the cooling curves for solution of tertbutyl alcohol and deionized water. If there are more times/entries than needed for the number of temperatures that you recorded, enter zero (0.00) for the remaining (25pts) Part B: 2. Calculate the molality of water in the solution used in Part B. Volume of deionized water added to test tube (mL) : Mass of graduated cylinder and alcohol (g) : Mass of empty graduated cylinder (g) : (0.5pts) Mass of alcohol (g) (3pts) Molality of water in the solution (m) (0.5pts) For full credit, upload an image of ALL your calculations for Part B question 2. Part B. Freezing point determination of solution of tert-butyl alcohol and deionized water Table view List view (1pts) Average freezing point for the solution of teri-butyl alcohol and deionized water ( C ) (2pts) Upload your graphs for Part B here. Make sure you have an appropriate titles, axis labels, and that the equations for the trendlines are displayed on the chart. You can upload your graphs directly (as a .Xlsx, csv, or .txt file) or upload a screen shot or photo of your graphs (as a .pdi, .jpg. .png, or heic file). Note: Hand drawn araphs are not accepted. (1pts) For full credit, upload an image of ALL your calculations for Part B question 3. 4. Calculate T for the solution in Part B. Remember T=TpureTsolution:. (3pts) T(C) (1pts) For full credit, upload an image of your calculation for Part B question 4. Browse your files to upload or Drag and Drop Max attachments: 5 I Max Size: 20.00MM each 5. Calculate the freezing point depression constant, Kf, for tert-butyl alcohol using the T calculated in question 4. (Apts) Kf(C/m) (1pts) For full credit, upload an image of your calculation for Part B question 5