Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part B is most important thank you!!! Copper is an essential trace element for biology and it is also used in many industrial applications. Copper
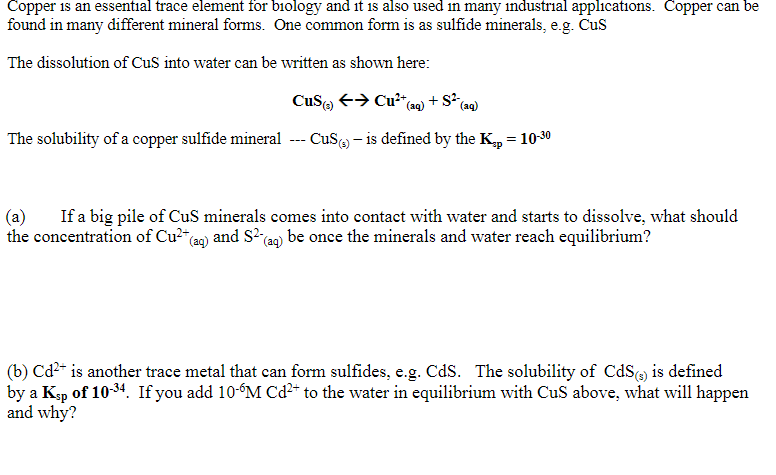
Part B is most important thank you!!!
Copper is an essential trace element for biology and it is also used in many industrial applications. Copper can be found in many different mineral forms. One common form is as sulfide minerals, e.g. Cus The dissolution of Cus into water can be written as shown here: Cusca + Cu+ (aq) + S2 (aq) The solubility of a copper sulfide mineral --- Cuso - is defined by the K.; = 10-30 = (a) If a big pile of CuS minerals comes into contact with water and starts to dissolve, what should the concentration of Cu2+(ag) and S2 (aq) be once the minerals and water reach equilibrium? (b) Cd- is another trace metal that can form sulfides, e.g. Cds. The solubility of CdS) is defined by a Ksp of 10-34. If you add 10-6M Cd2+ to the water in equilibrium with CuS above, what will happen and whyStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


