Answered step by step
Verified Expert Solution
Question
1 Approved Answer
part b, please! 3.1 Two moles of nitrogen are initially at 10 bar and 600 K (state 1) in a horizontal pistom/cylin der device. They
part b, please! 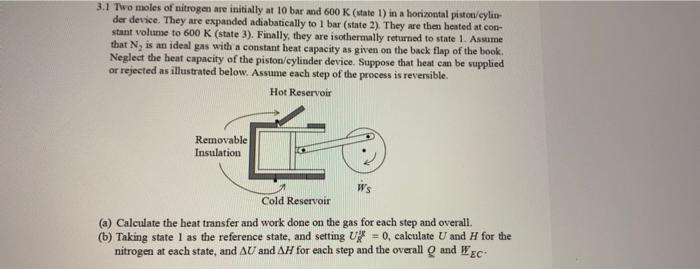
3.1 Two moles of nitrogen are initially at 10 bar and 600 K (state 1) in a horizontal pistom/cylin der device. They are expanded adiabatically to 1 bar (state 2). They are the heated at con stant volume to 600 K (state 3). Finally, they are isothermally returned to state 1. Assume that N, is an ideal gas with a constant heat capacity as given on the back flap of the book, Neglect the heat capacity of the piston/cylinder device. Suppose that heat can be supplied or rejected as illustrated below. Assume each step of the process is reversible. Hot Reservoir Removable Insulation WS Cold Reservoir (a) Calculate the heat transfer and work done on the gas for each step and overall. (b) Taking state 1 as the reference state, and setting U = 0, calculate U and H for the nitrogen at each state, and AU and A4 for each step and the overall and Wec 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


