Answered step by step
Verified Expert Solution
Question
1 Approved Answer
part b pls!! You wish to determine the activation energy for the following first-order reaction: AB+C Part A What data would you collect? Measure the
part b pls!! 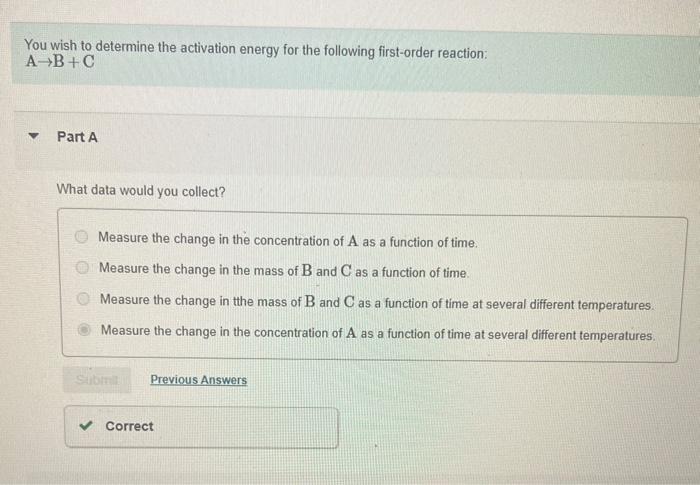
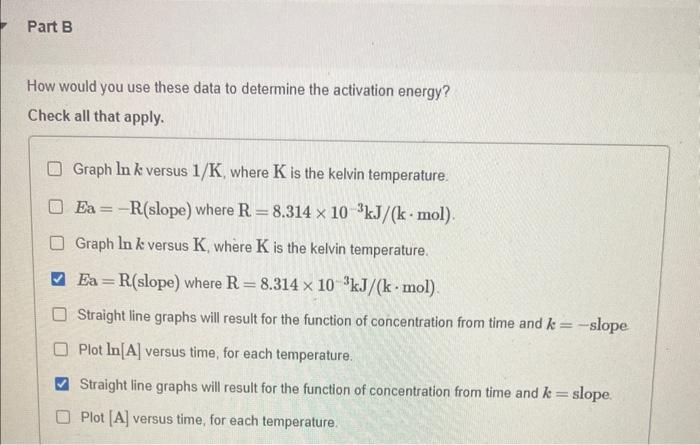
You wish to determine the activation energy for the following first-order reaction: AB+C Part A What data would you collect? Measure the change in the concentration of A as a function of time. Measure the change in the mass of B and C as a function of time. Measure the change in the mass of B and C as a function of time at several different temperatures. Measure the change in the concentration of A as a function of time at several different temperatures. How would you use these data to determine the activation energy? Check all that apply. Graph lnk versus 1/K, where K is the kelvin temperature. Ea=R( slope ) where R=8.314103kJ/(kmol). Graph lnk versus K, where K is the kelvin temperature. Ea=R (slope) where R=8.314103kJ/(kmol). Straight line graphs will result for the function of concentration from time and k= slope Plot ln[A] versus time, for each temperature. Straight line graphs will result for the function of concentration from time and k= slope. Plot [A] versus time, for each temperature 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


