Answered step by step
Verified Expert Solution
Question
1 Approved Answer
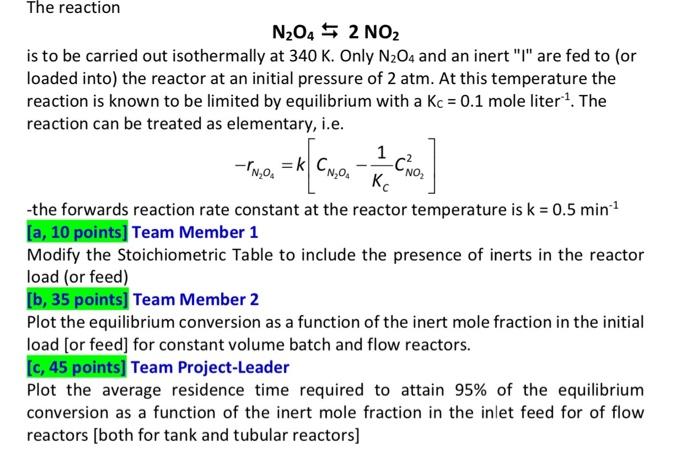
part c please =k CN,O -CNO The reaction N204 2 NO2 is to be carried out isothermally at 340 K. Only N204 and an inert
part c please
=k CN,O -CNO The reaction N204 2 NO2 is to be carried out isothermally at 340 K. Only N204 and an inert "l" are fed to (or loaded into) the reactor at an initial pressure of 2 atm. At this temperature the reaction is known to be limited by equilibrium with a Kc = 0.1 mole liter1. The reaction can be treated as elementary, i.e. 1 -NOA -the forwards reaction rate constant at the reactor temperature is k = 0.5 min-1 [a, 10 points] Team Member 1 Modify the Stoichiometric Table to include the presence of inerts in the reactor load (or feed) [b, 35 points) Team Member 2 Plot the equilibrium conversion as a function of the inert mole fraction in the initial load for feed] for constant volume batch and flow reactors. [C, 45 points] Team Project-Leader Plot the average residence time required to attain 95% of the equilibrium conversion as a function of the inert mole fraction in the inlet feed for of flow reactors [both for tank and tubular reactors) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


