Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PART C: Problems (20 marks) Answer all questions in the space provided. Show complete solutions for full marks. All answers must be expressed using
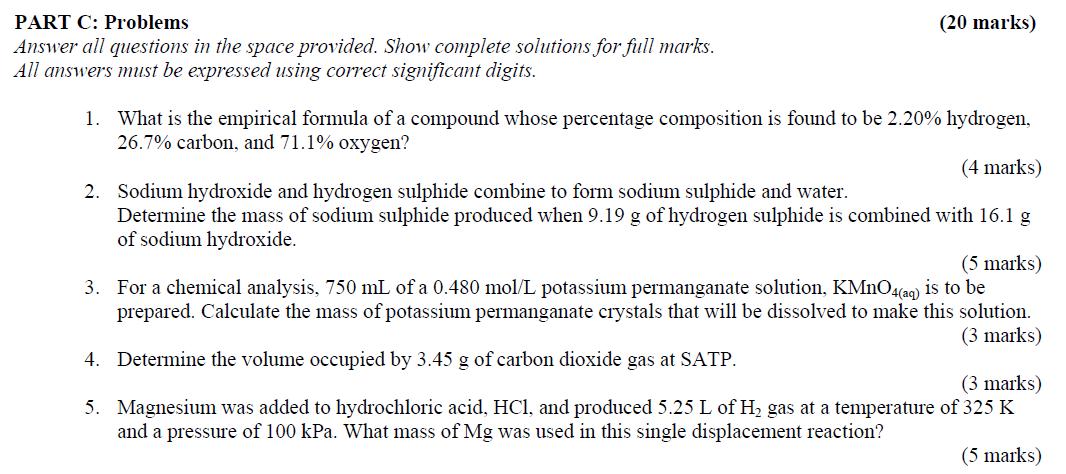
PART C: Problems (20 marks) Answer all questions in the space provided. Show complete solutions for full marks. All answers must be expressed using correct significant digits. 1. What is the empirical formula of a compound whose percentage composition is found to be 2.20% hydrogen, 26.7% carbon, and 71.1% oxygen? (4 marks) 2. Sodium hydroxide and hydrogen sulphide combine to form sodium sulphide and water. Determine the mass of sodium sulphide produced when 9.19 g of hydrogen sulphide is combined with 16.1 g of sodium hydroxide. (5 marks) 3. For a chemical analysis, 750 mL of a 0.480 mol/L potassium permanganate solution, KMnO4(caq) is to be prepared. Calculate the mass of potassium permanganate crystals that will be dissolved to make this solution. (3 marks) 4. Determine the volume occupied by 3.45 g of carbon dioxide gas at SATP. (3 marks) 5. Magnesium was added to hydrochloric acid, HCI, and produced 5.25 L of H2 gas at a temperature of 325 K and a pressure of 100 kPa. What mass of Mg was used in this single displacement reaction? (5 marks)
Step by Step Solution
★★★★★
3.48 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


