Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Partial and total vapor pressures for methanol (Component B) and water (Component C) solution at 40C is given in the Figure 1 below. e)
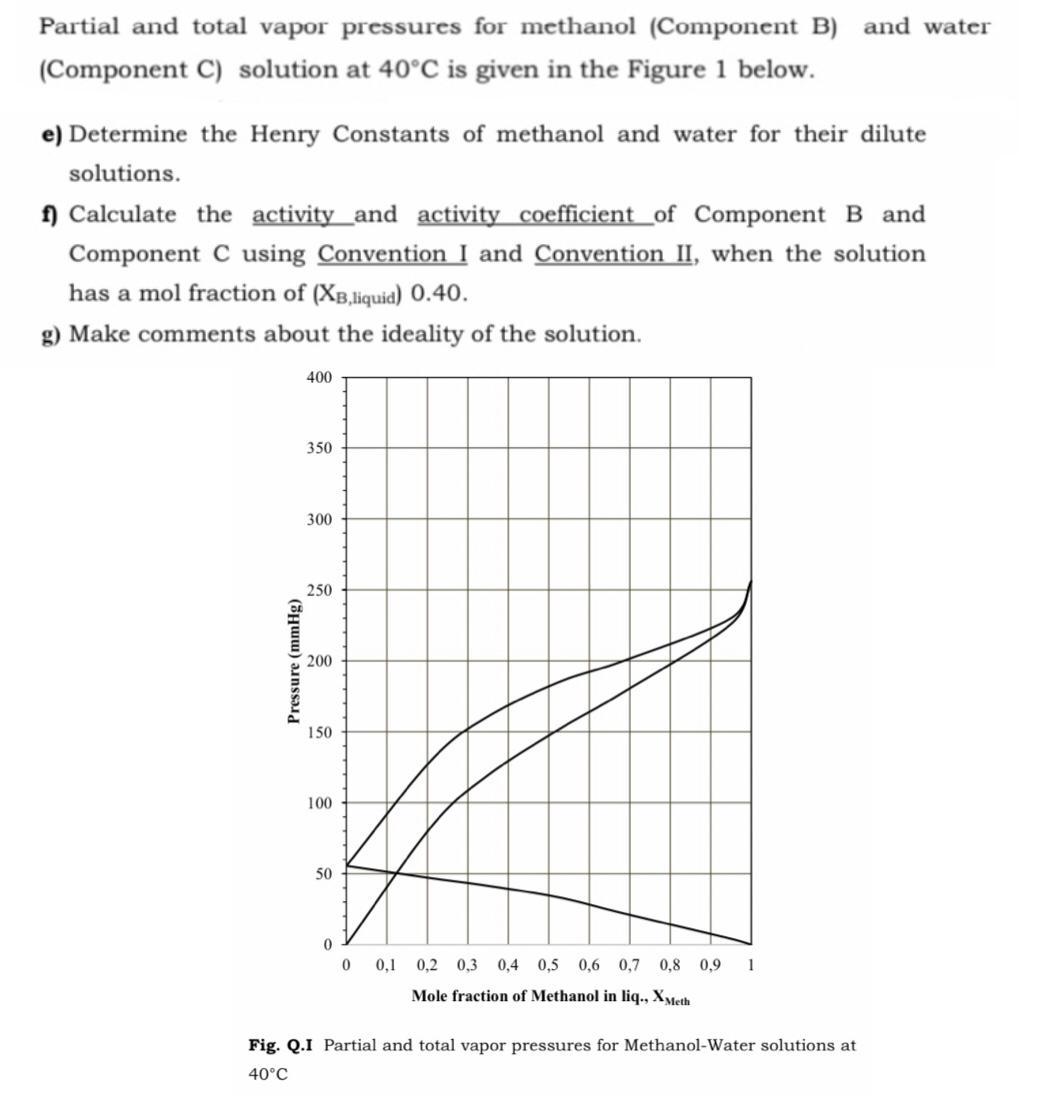
Partial and total vapor pressures for methanol (Component B) and water (Component C) solution at 40C is given in the Figure 1 below. e) Determine the Henry Constants of methanol and water for their dilute solutions. 1 Calculate the activity _and activity coefficient_of Component B and Component C using Convention I and Convention II, when the solution has a mol fraction of (XB,iquid) 0.40. g) Make comments about the ideality of the solution. 400 350 300 250 200 150 100 50 0,1 0,2 0,3 0,4 0,5 0,6 0,7 0,8 0,9 1 Mole fraction of Methanol in liq., Xyeth Fig. Q.I Partial and total vapor pressures for Methanol-Water solutions at 40C Pressure (mmHg)
Step by Step Solution
★★★★★
3.44 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
63636c77105da_230082.pdf
180 KBs PDF File
63636c77105da_230082.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


