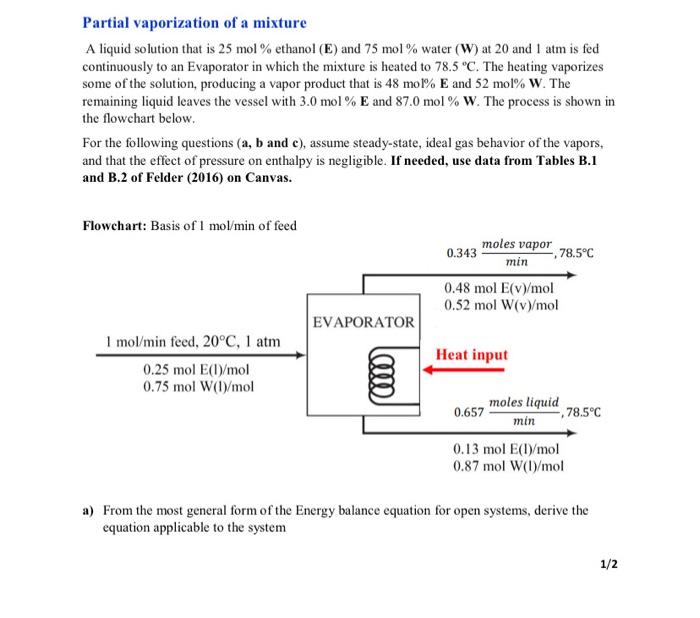
Partial vaporization of a mixture A liquid solution that is 25mol% ethanol (E) and 75mol% water (W) at 20 and 1atm is fed continuously to an Evaporator in which the mixture is heated to 78.5C. The heating vaporizes some of the solution, producing a vapor product that is 48mol%E and 52mol%W. The remaining liquid leaves the vessel with 3.0mol%E and 87.0mol%W. The process is shown in the flowchart below. For the following questions (a, b and c ), assume steady-state, ideal gas behavior of the vapors, and that the effect of pressure on enthalpy is negligible. If needed, use data from Tables B.1 and B.2 of Felder (2016) on Canvas. a) From the most general form of the Energy balance equation for open systems, derive the equation applicable to the system b) The Enthalpy Table for the process is given below Reference states: E(l,20C,1 atm), W(l,20C,1 atm) Calculate the specific enthalpies of the output in the Enthalpy Table: H1,H2,H3 and H4 (Ans: H1=7.64kJ/mol,H^2=4.41kJ/mol,H^3=46.22kj/mol and H^4=45.95kJ/mol ) Hint: To get H^i for each substance, make a path from the reference state to the final state (See our examples discussed in class). As an example, the solution for H^1 is given below: Path to get H^1:E(I) at 20C,1atmE(I) at 78.5C,P of output Neglecting the effect of pressure: H^1=2078.5(Cp)(t)dT From B.2, the (Cp)E()(kJ/mol(C) is given at two temperatures: 0.1031 at 0C and 0.1588 at 100C. We estimate the (Cp)E(0) at 49.25C using linear interpolation: The slope through the points is assumed to be equal (linear relationship): 10000.15880.1031=49.250(Cp)E(D)0.1031,and(Cp)E(t)aat49C=0.1305kl//molCCH^1=20C7.5C(0.1305)dT=(0.1305)(78.520)=7.64kJ/mol c) Using the Energy balance equation from (a), solve for the rate at which heat must be added to the system. (Ans: Q=18.97kJ/min ) Partial vaporization of a mixture A liquid solution that is 25mol% ethanol (E) and 75mol% water (W) at 20 and 1atm is fed continuously to an Evaporator in which the mixture is heated to 78.5C. The heating vaporizes some of the solution, producing a vapor product that is 48mol%E and 52mol%W. The remaining liquid leaves the vessel with 3.0mol%E and 87.0mol%W. The process is shown in the flowchart below. For the following questions (a, b and c ), assume steady-state, ideal gas behavior of the vapors, and that the effect of pressure on enthalpy is negligible. If needed, use data from Tables B.1 and B.2 of Felder (2016) on Canvas. a) From the most general form of the Energy balance equation for open systems, derive the equation applicable to the system b) The Enthalpy Table for the process is given below Reference states: E(l,20C,1 atm), W(l,20C,1 atm) Calculate the specific enthalpies of the output in the Enthalpy Table: H1,H2,H3 and H4 (Ans: H1=7.64kJ/mol,H^2=4.41kJ/mol,H^3=46.22kj/mol and H^4=45.95kJ/mol ) Hint: To get H^i for each substance, make a path from the reference state to the final state (See our examples discussed in class). As an example, the solution for H^1 is given below: Path to get H^1:E(I) at 20C,1atmE(I) at 78.5C,P of output Neglecting the effect of pressure: H^1=2078.5(Cp)(t)dT From B.2, the (Cp)E()(kJ/mol(C) is given at two temperatures: 0.1031 at 0C and 0.1588 at 100C. We estimate the (Cp)E(0) at 49.25C using linear interpolation: The slope through the points is assumed to be equal (linear relationship): 10000.15880.1031=49.250(Cp)E(D)0.1031,and(Cp)E(t)aat49C=0.1305kl//molCCH^1=20C7.5C(0.1305)dT=(0.1305)(78.520)=7.64kJ/mol c) Using the Energy balance equation from (a), solve for the rate at which heat must be added to the system. (Ans: Q=18.97kJ/min )