Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Particle #2 - Formaldehyde molecule ( CH2O) Question 26 - 30 This molecule has three bonds: two CH and one CO bonds. Answer the following
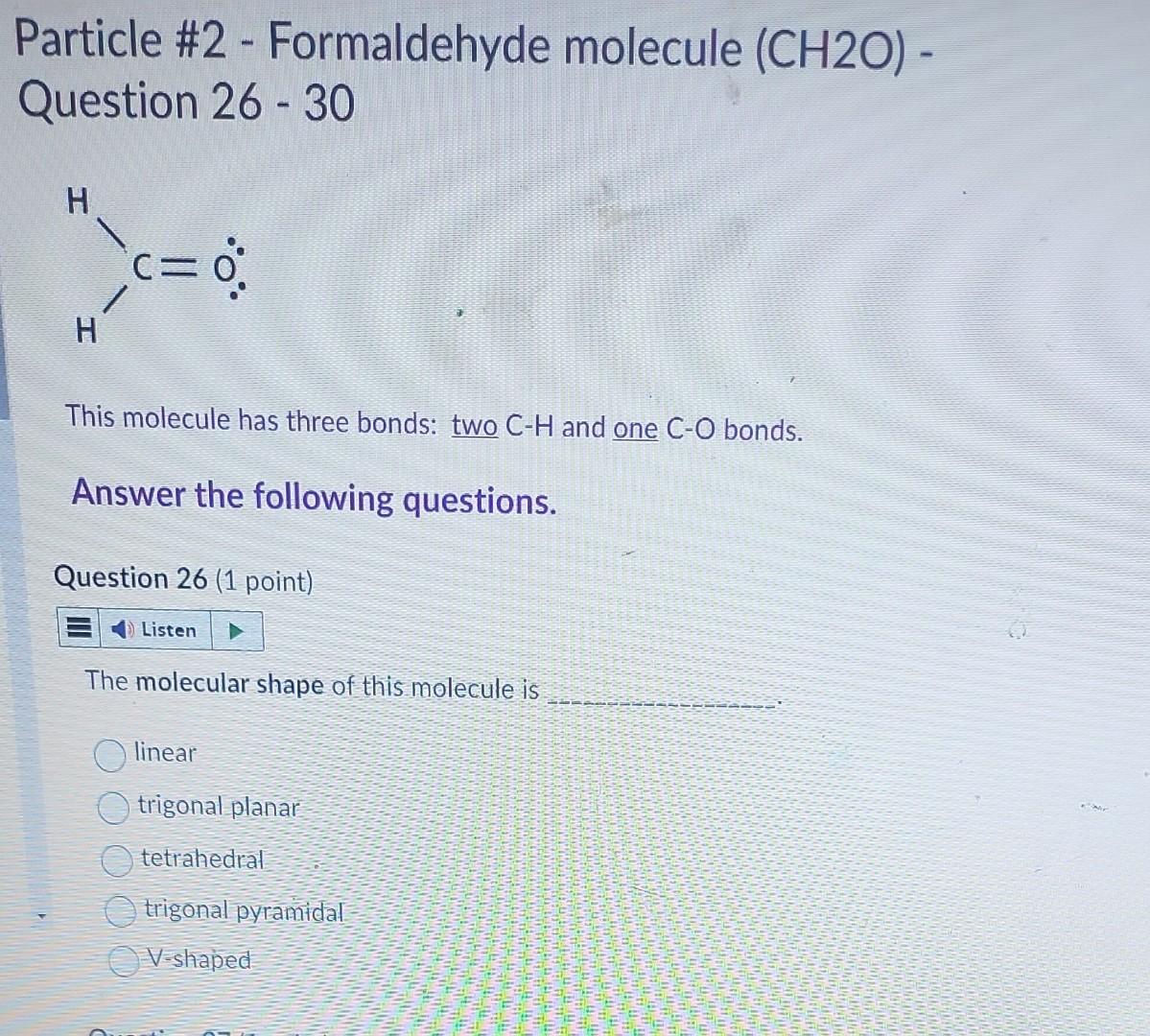
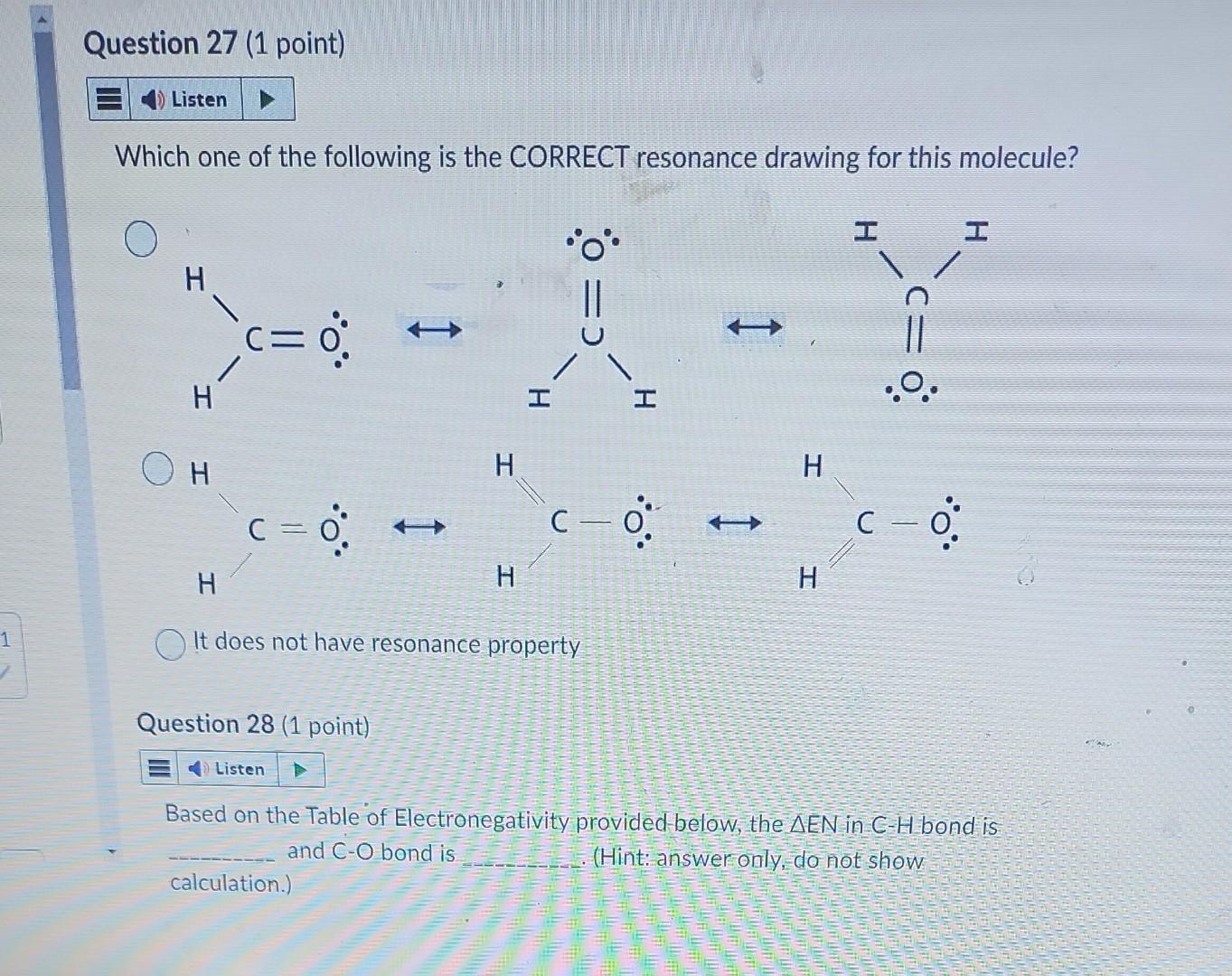
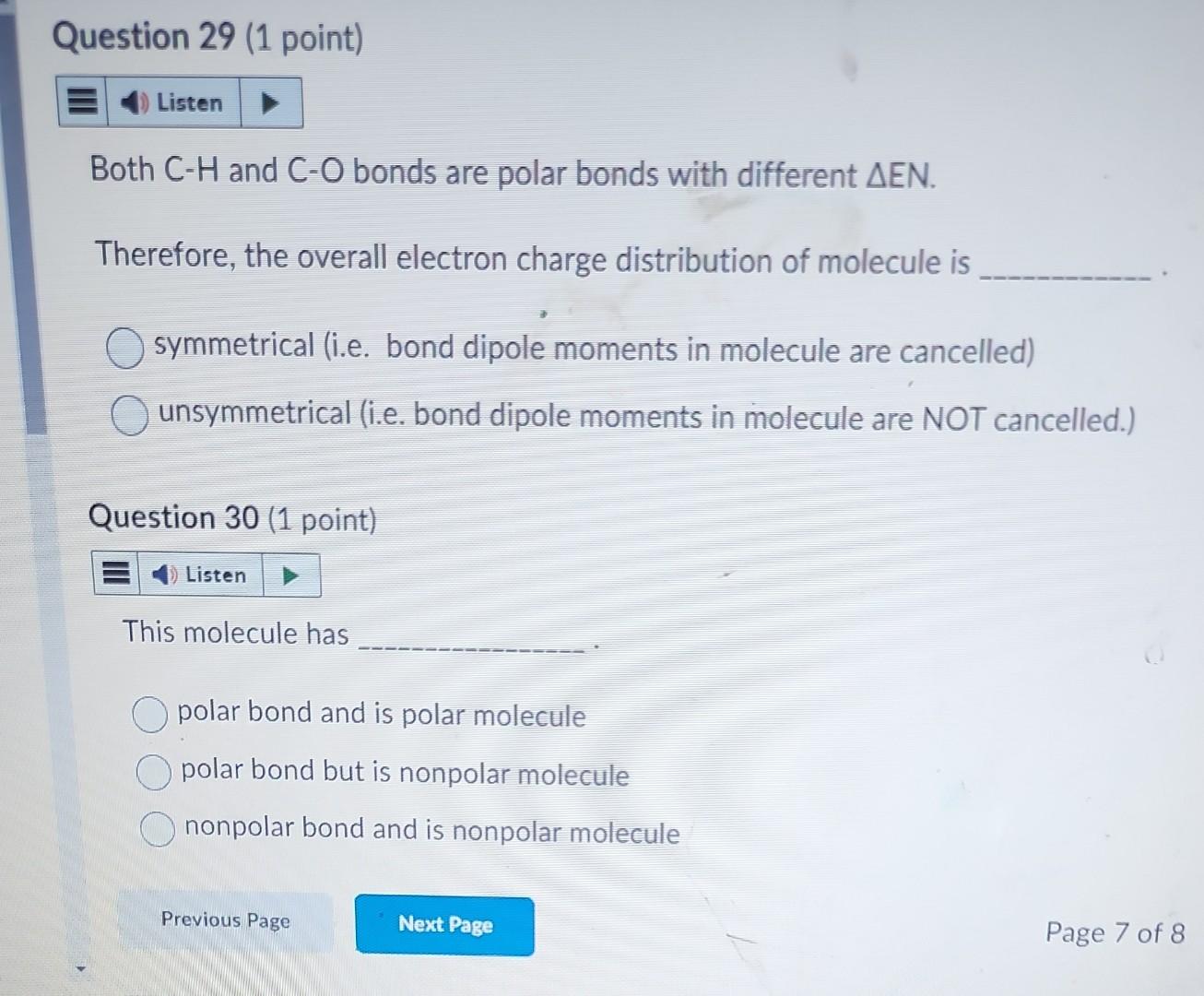
Particle \#2 - Formaldehyde molecule ( CH2O) Question 26 - 30 This molecule has three bonds: two CH and one CO bonds. Answer the following questions. Question 26 (1 point) The molecular shape of this molecule is linear trigonal planar tetrahedral trigonal pyramidal V-shaped Which one of the following is the CORRECT resonance drawing for this molecule? It does not have resonance property Question 28 (1 point) Based on the Table of Electronegativity provided-below, the EN in CH bond is and CO bond is (Hint: answer only, do not show calculation.) Both CH and CO bonds are polar bonds with different EN. Therefore, the overall electron charge distribution of molecule is symmetrical (i.e. bond dipole moments in molecule are cancelled) unsymmetrical (i.e. bond dipole moments in molecule are NOT cancelled.) Question 30 (1 point) This molecule has polar bond and is polar molecule polar bond but is nonpolar molecule nonpolar bond and is nonpolar molecule
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


