Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Parts a, b, c, and d please 2. This question concerns visual depiction of the wave functions, probabilities, and energy levels of a 1D particle
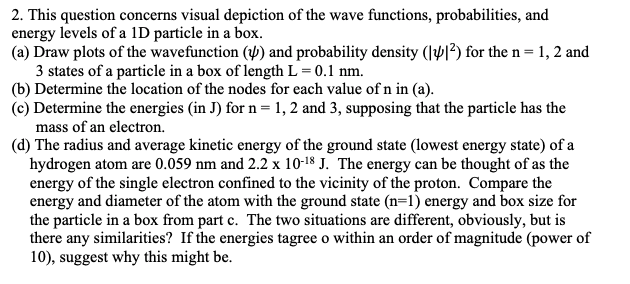
Parts a, b, c, and d please
2. This question concerns visual depiction of the wave functions, probabilities, and energy levels of a 1D particle in a box. (a) Draw plots of the wavefunction () and probability density (2) for the n=1,2 and 3 states of a particle in a box of length L=0.1nm. (b) Determine the location of the nodes for each value of n in (a). (c) Determine the energies (in J) for n=1,2 and 3, supposing that the particle has the mass of an electron. (d) The radius and average kinetic energy of the ground state (lowest energy state) of a hydrogen atom are 0.059nm and 2.21018J. The energy can be thought of as the energy of the single electron confined to the vicinity of the proton. Compare the energy and diameter of the atom with the ground state (n=1) energy and box size for the particle in a box from part c. The two situations are different, obviously, but is there any similarities? If the energies tagree o within an order of magnitude (power of 10), suggest why this might beStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


