Answered step by step
Verified Expert Solution
Question
1 Approved Answer
parts e-h only please! thank you! (20 points) Condensation Follow the standard energy balance steps to determine the water condensed and the heat removed from
parts e-h only please! thank you! 

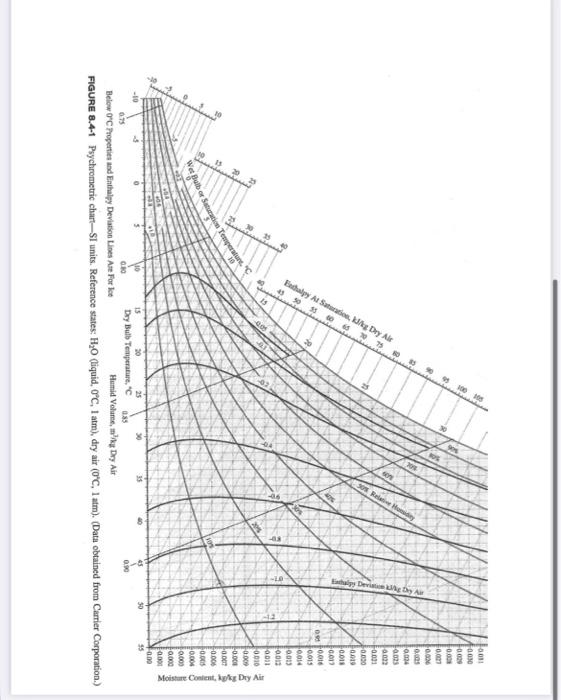
(20 points) Condensation Follow the standard energy balance steps to determine the water condensed and the heat removed from a humid air stream. The feed stream contains 15 wt% water (balance is air), has a temperature of 200C and a pressure of 1 atm. The exit gas stream is at 1 atm and 30C. The exit vapor composition should be determined from the psychrometric chart (saturated). The heat of vaporization of water at 30C is 43.8 kJ/mol. a. Draw a picture and label appropriately (Basis: 1 kg/s feed). b. Perform a degree of freedom analysis. c. Write appropriate equations. d. Complete all material balances. e. Specify reference states. f. Construct and fill out enthalpy table. Define variables for unknown enthalpies. Species/Phase min (kg/s) In (kJ/mol) mout (kg/s) H:0 (v) Air (g) H:O (1) g. Determine unknown enthalpies. h. Determine Q. Please give your answer in kW. Hout (kJ/mol) 20 15 75 70 Enthalpy At Saturation, kJ/kg Dry Air 45 50 55 60 65 059095505 15 A 60 83 90 95 100 105 0.91 00: 0.030 0000 0.008 0.007 0.006 0.025 0,024 Ea025 0.022 -0.001 -0.000 0.019 -0.018 0.017 0.016 0.015 0.014 0.013 0.012 0011 0.010 0.009 -0.008 -0.007 -0.006 0.005 0.004 0.000 0.000 -0.000 0.00 Moisture Content, kg/kg Dry Air C 10 Wet Balbor Saturation Temperature. 0.75 15 20 Dry Bulb Temperature, "C 25 0.80 30 Below 0C Properties and Enthalpy Deviation Lines Are For Ice FIGURE 8.4-1 Psychrometric chart-SI units. Reference states: HO (liquid, 0C, 1 atm), dry air (0C, 1 atm). (Data obtained from Carrier Corporation.) 30 0.85 Humid Volume, m'/kg Dry Air 55 0.90 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


