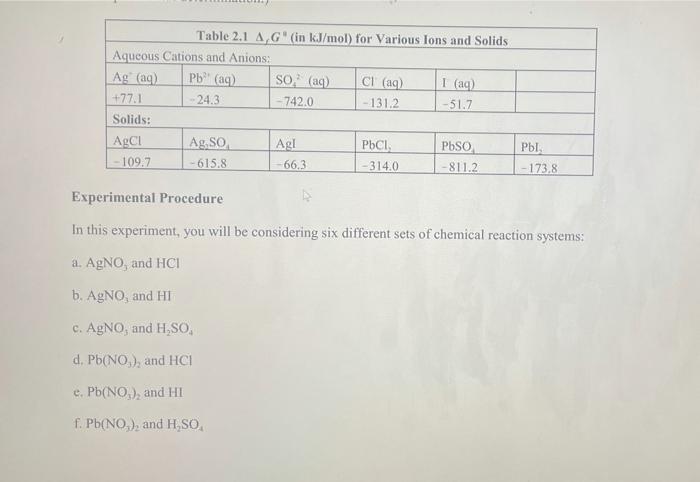
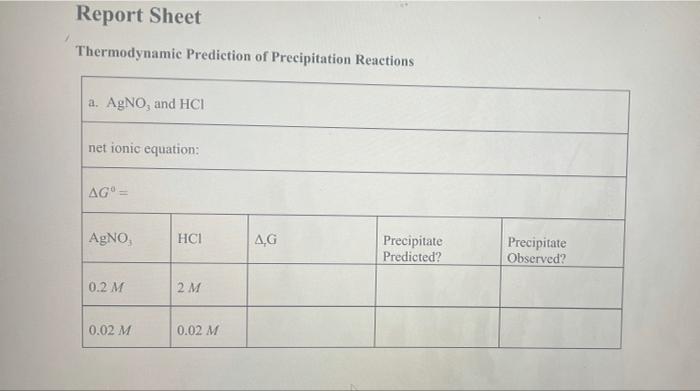
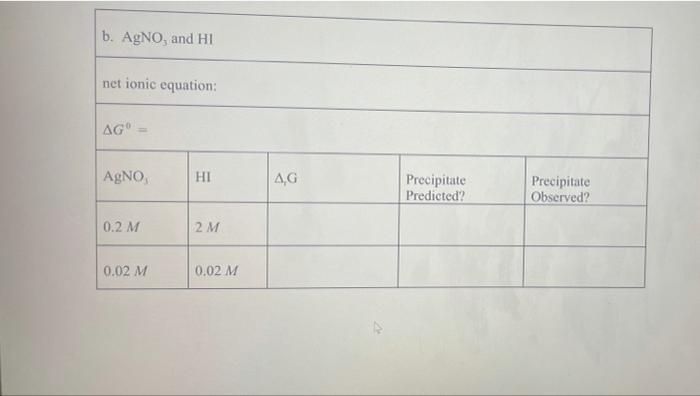
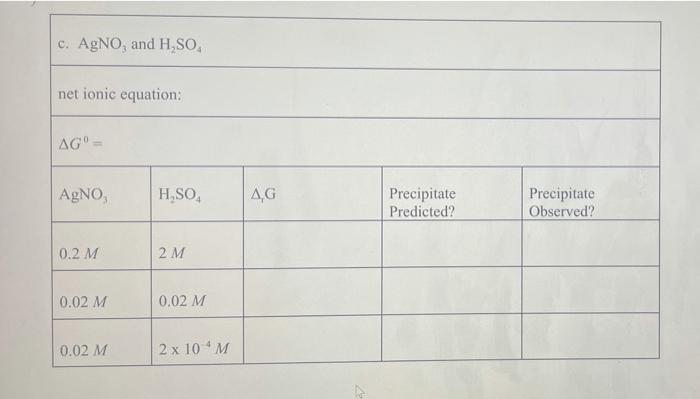
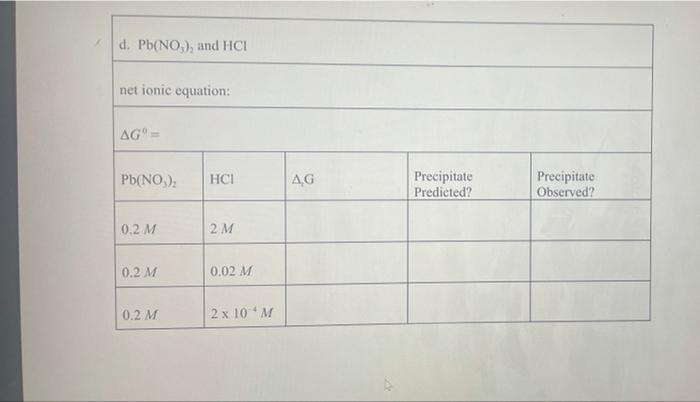
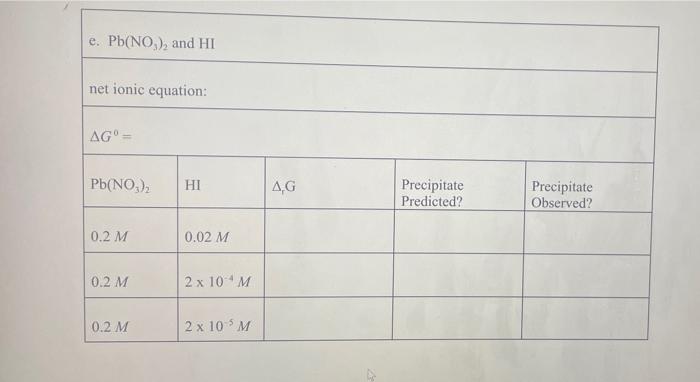
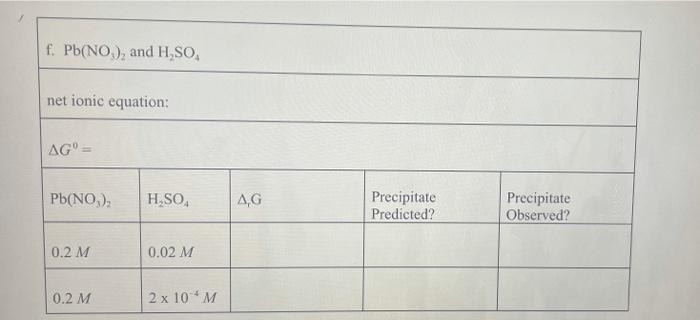
Pb (a Cl(aq) I (aq) Table 2.1 AG (in kJ/mol) for Various lons and Solids Aqueous Cations and Anions Ag (aq) SO, (aq) +77.1 24.3 742.0 - 131.2 51.7 Solids: AgCl Ag SO Ag! PbCI. PbSO - 109.7 -615.8 66.3 314.0 -811.2 , 173.8 Experimental Procedure In this experiment, you will be considering six different sets of chemical reaction systems: a. AgNO, and HCI b. AgNO, and HD c. AgNO, and H.SO, d. Pb(NO), and HCI e. Pb(NO), and HD f. Pb(NO), and H.SO. Report Sheet Thermodynamic Prediction of Precipitation Reactions 2. AgNO, and HCI net ionic equation: AG AgNO HCl AG Precipitate Predicted? Precipitate Observed? 0.2 M 2M 0.02 M 0.02 M b. AgNO, and HI net ionic equation: AG AgNO, HI AG Precipitate Predicted? Precipitate Observed? 0.2 M 2M 0.02 M 0.02 M c. AgNO, and H SO. net ionic equation: AG" AgNO H.SO AG Precipitate Predicted? Precipitate Observed? 0.2 M 2M 0.02 M 0.02 M 0.02 M 2 x 10M d. Pb(NO), and HCI net ionic equation: AG" = Pb(NO), AG Precipitate Predicted? Precipitate Observed? 0.2 M 2M 0.2 M 0.02 M 0.2 M 2 x 10 M e. Pb(NO), and HI net ionic equation: AG Pb(NO) HI .G Precipitate Predicted? Precipitate Observed? 0.2 M 0.02 M 0.2 M 2 x 10 M 0.2 M 2 x 10 M f. Pb(NO), and H.SO. net ionic equation: AG" Pb(NO) H SO | AG Precipitate Predicted? Precipitate Observed? 0.2 M 0.02 M 0.2 M 2 x 10 *M Pb (a Cl(aq) I (aq) Table 2.1 AG (in kJ/mol) for Various lons and Solids Aqueous Cations and Anions Ag (aq) SO, (aq) +77.1 24.3 742.0 - 131.2 51.7 Solids: AgCl Ag SO Ag! PbCI. PbSO - 109.7 -615.8 66.3 314.0 -811.2 , 173.8 Experimental Procedure In this experiment, you will be considering six different sets of chemical reaction systems: a. AgNO, and HCI b. AgNO, and HD c. AgNO, and H.SO, d. Pb(NO), and HCI e. Pb(NO), and HD f. Pb(NO), and H.SO. Report Sheet Thermodynamic Prediction of Precipitation Reactions 2. AgNO, and HCI net ionic equation: AG AgNO HCl AG Precipitate Predicted? Precipitate Observed? 0.2 M 2M 0.02 M 0.02 M b. AgNO, and HI net ionic equation: AG AgNO, HI AG Precipitate Predicted? Precipitate Observed? 0.2 M 2M 0.02 M 0.02 M c. AgNO, and H SO. net ionic equation: AG" AgNO H.SO AG Precipitate Predicted? Precipitate Observed? 0.2 M 2M 0.02 M 0.02 M 0.02 M 2 x 10M d. Pb(NO), and HCI net ionic equation: AG" = Pb(NO), AG Precipitate Predicted? Precipitate Observed? 0.2 M 2M 0.2 M 0.02 M 0.2 M 2 x 10 M e. Pb(NO), and HI net ionic equation: AG Pb(NO) HI .G Precipitate Predicted? Precipitate Observed? 0.2 M 0.02 M 0.2 M 2 x 10 M 0.2 M 2 x 10 M f. Pb(NO), and H.SO. net ionic equation: AG" Pb(NO) H SO | AG Precipitate Predicted? Precipitate Observed? 0.2 M 0.02 M 0.2 M 2 x 10 *M