Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Percent error can be used to calculate the amount of error in an experimental or measured value comparing it to a literature or expected
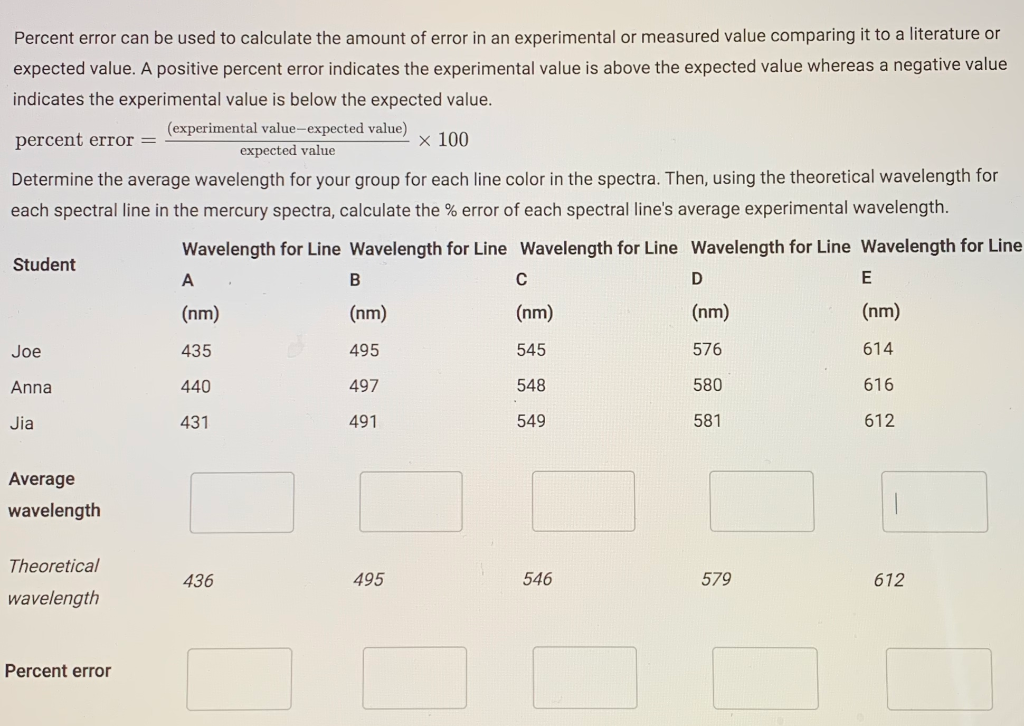
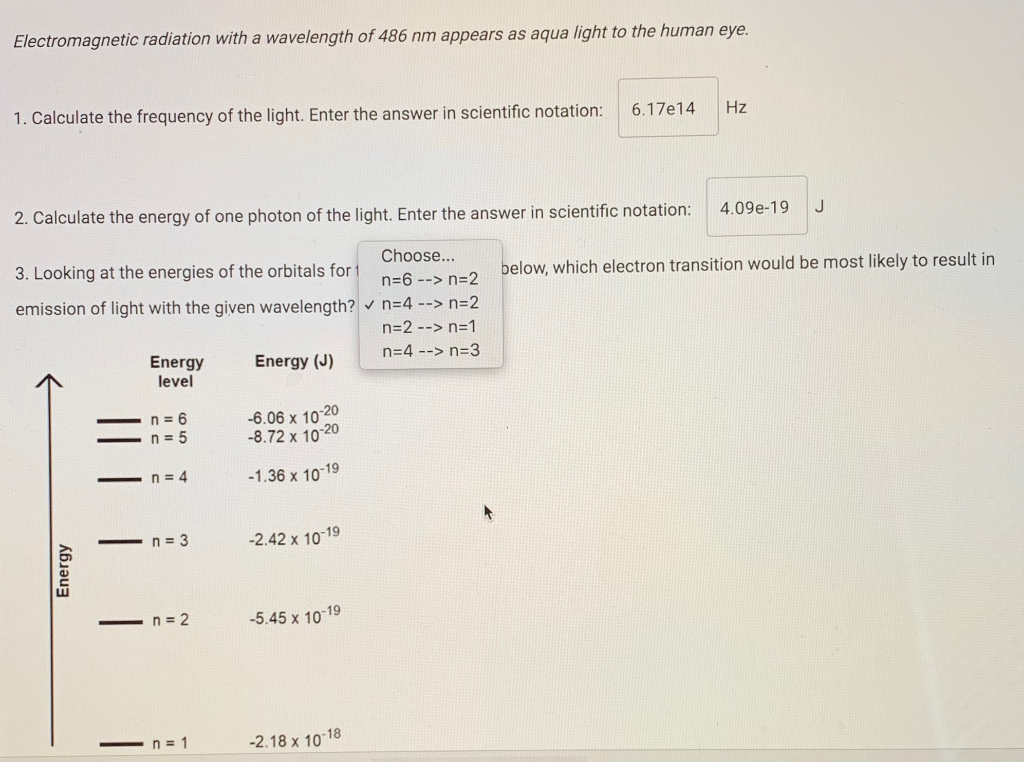
Percent error can be used to calculate the amount of error in an experimental or measured value comparing it to a literature or expected value. A positive percent error indicates the experimental value is above the expected value whereas a negative value indicates the experimental value is below the expected value. percent error = x 100 Determine the average wavelength for your group for each line color in the spectra. Then, using the theoretical wavelength for each spectral line in the mercury spectra, calculate the % error of each spectral line's average experimental wavelength. Wavelength for Line Wavelength for Line Wavelength for Line Wavelength for Line Wavelength for Line Student Joe Annal Jia Average wavelength Theoretical wavelength (experimental value-expected value) expected value Percent error A (nm) 435 440 431 436 B (nm) 495 497 491 495 C (nm) 545 548 549 546 D (nm) 576 580 581 579 E (nm) 614 616 612 612 Electromagnetic radiation with a wavelength of 486 nm appears as aqua light to the human eye. 1. Calculate the frequency of the light. Enter the answer in scientific notation: 2. Calculate the energy of one photon of the light. Enter the answer in scientific notation: Choose... n=6 --> n=2 n=4 --> n=2 n=2 --> n=1 n=4 --> n=3 3. Looking at the energies of the orbitals for t emission of light with the given wavelength? Energy Energy level n = 6 n = 5 n = 4 n = 3 n = 2 n = 1 Energy (J) -6.06 x 10-20 -8.72 x 10-20 -1.36 x 10-19 -2.42 x 10-19 -5.45 x 10-19 -2.18 x 10-18 6.17e14 Hz F 4.09e-19 J below, which electron transition would be most likely to result in
Step by Step Solution
★★★★★
3.50 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
The average wavelengths can be calculated as the sum af all values ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


