Question
What is the hybridization at the indicated atom in the molecules and ions listed below? Use a wedge-dash drawing, if appropriate, and show all
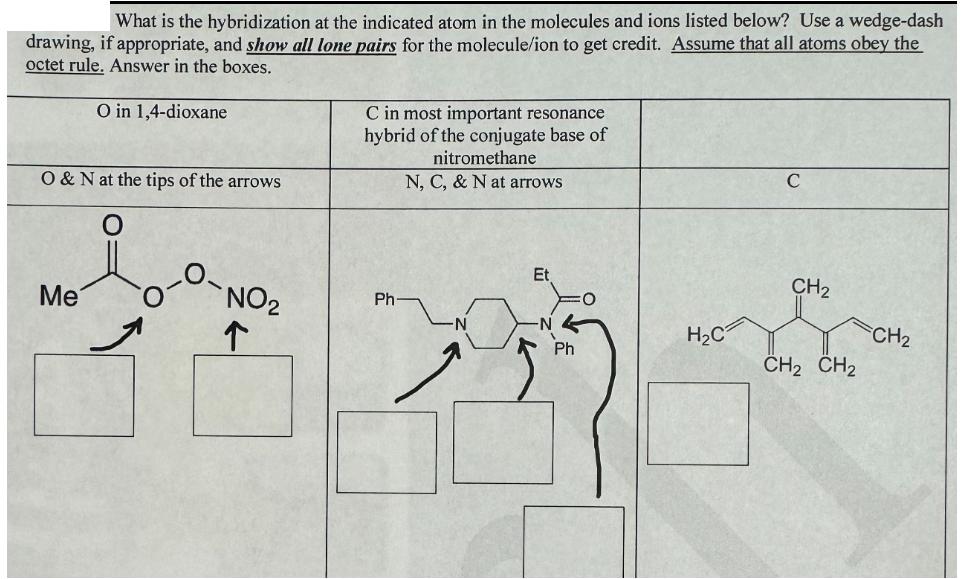
What is the hybridization at the indicated atom in the molecules and ions listed below? Use a wedge-dash drawing, if appropriate, and show all lone pairs for the molecule/ion to get credit. Assume that all atoms obey the octet rule. Answer in the boxes. O in 1,4-dioxane O & N at the tips of the arrows C in most important resonance hybrid of the conjugate base of nitromethane N, C, & N at arrows Me NO2 Ph Et CH2 HC CH2 Ph CH2 CH2
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
General guidelines Follow the steps kindly O in 14dioxane part has been solved belo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Communication Essentials a skill based approach
Authors: Courtland L. Bovee, John V. Thill
6th edition
978-0132971324
Students also viewed these Programming questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



