Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Photoelectron spectroscopy typically measures the binding energy of electrons in units of MJ/mol. Use the value for the energy of an electron in a hydrogen
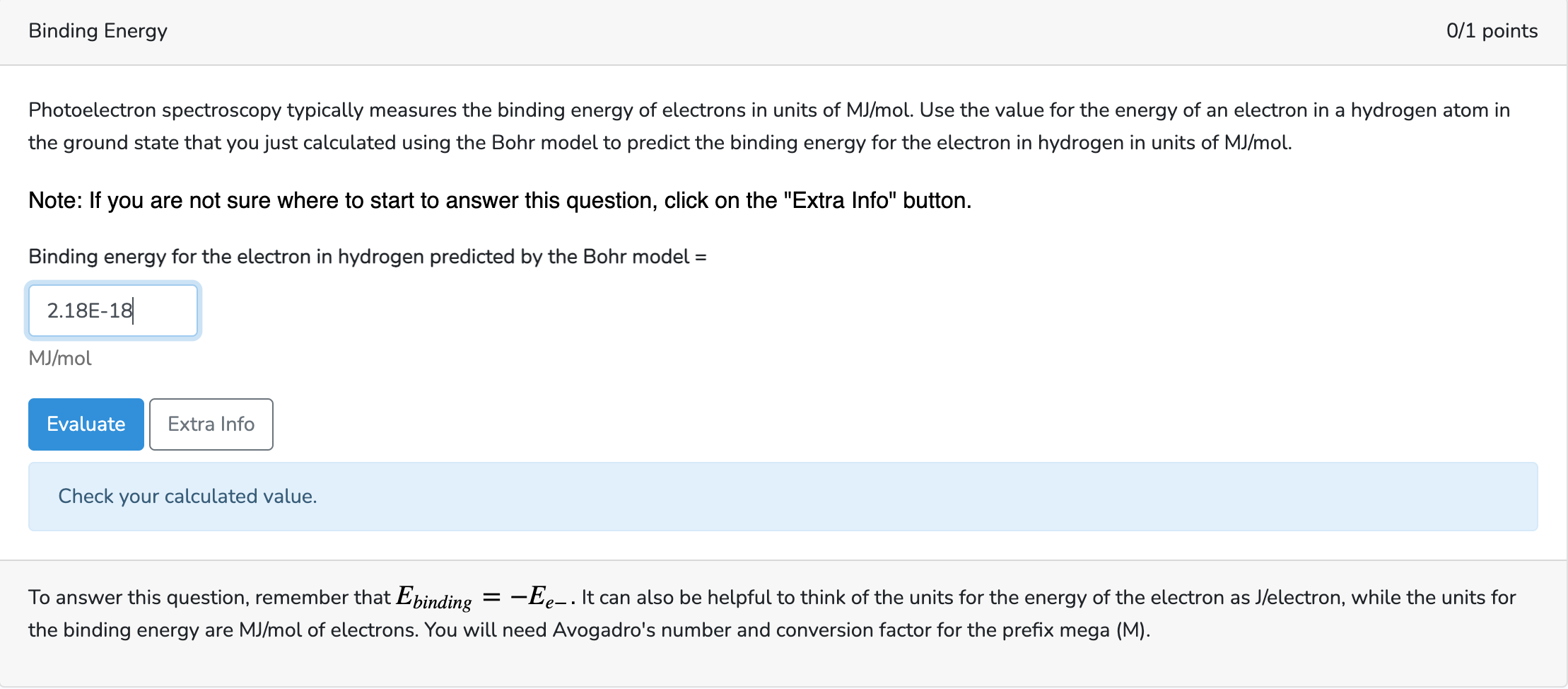 Photoelectron spectroscopy typically measures the binding energy of electrons in units of MJ/mol. Use the value for the energy of an electron in a hydrogen atom in the ground state that you just calculated using the Bohr model to predict the binding energy for the electron in hydrogen in units of MJ/mol. Note: If you are not sure where to start to answer this question, click on the "Extra Info" button. Binding energy for the electron in hydrogen predicted by the Bohr model =
Photoelectron spectroscopy typically measures the binding energy of electrons in units of MJ/mol. Use the value for the energy of an electron in a hydrogen atom in the ground state that you just calculated using the Bohr model to predict the binding energy for the electron in hydrogen in units of MJ/mol. Note: If you are not sure where to start to answer this question, click on the "Extra Info" button. Binding energy for the electron in hydrogen predicted by the Bohr model =
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


