Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PHYSICAL CHEMISTRY PLS HELP ASAP Table 1 shows the concentration of reactant P at various time during the decomposition of P to product Q and
PHYSICAL CHEMISTRY PLS HELP ASAP 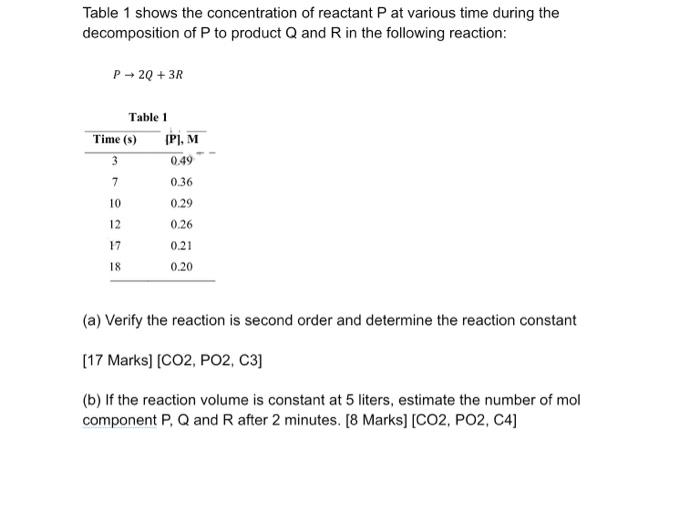
Table 1 shows the concentration of reactant P at various time during the decomposition of P to product Q and R in the following reaction: P2Q + 3R Table 1 Time (8) IP,M 3 0.49 7 0.36 10 0.29 12 0.26 0.21 17 18 0.20 (a) Verify the reaction is second order and determine the reaction constant [17 Marks) (CO2, PO2, C3] (b) If the reaction volume is constant at 5 liters, estimate the number of mol component P, Q and R after 2 minutes. [8 Marks) (CO2, PO2, C4) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


