Answered step by step
Verified Expert Solution
Question
1 Approved Answer
physical chemistry question from Ira Levine 6th edition Chapter 16.62 SOLUTION: Ea=162kJ/mol A=7.0x10^10dm^3/mol.s How do I get to these results? Rate constants for the gas-phase
physical chemistry question from Ira Levine 6th edition Chapter 16.62
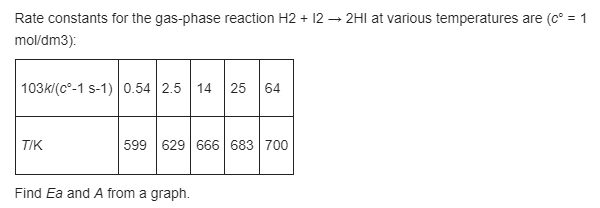
SOLUTION: Ea=162kJ/mol A=7.0x10^10dm^3/mol.s How do I get to these results?
Rate constants for the gas-phase reaction H2 + 12 2H1 at various temperatures are (C = 1 mol/dm3): 103k/(c-1 S-1) 0.54 2.5 14 25 64 TIK 599 629 666 | 683 700 Find Ea and A from a graphStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


