Question
A new potential heart medicine, code-named X-281, is being tested by a pharmaceutical company, Pharma-pill. As a research technician at Pharma-pill, you are tolo
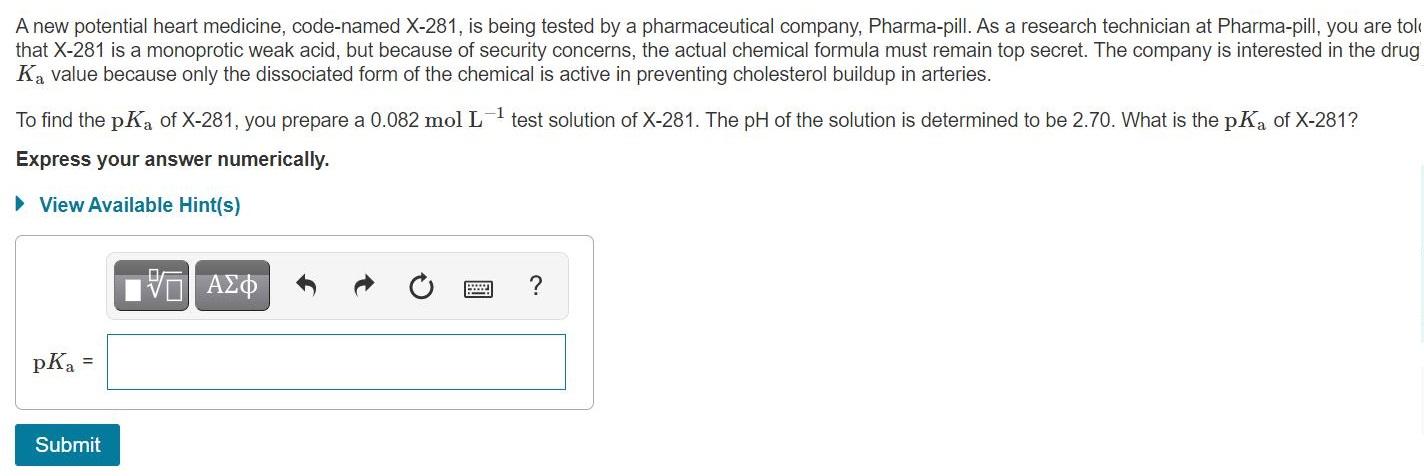
A new potential heart medicine, code-named X-281, is being tested by a pharmaceutical company, Pharma-pill. As a research technician at Pharma-pill, you are tolo that X-281 is a monoprotic weak acid, but because of security concerns, the actual chemical formula must remain top secret. The company is interested in the drug Ka value because only the dissociated form of the chemical is active in preventing cholesterol buildup in arteries. To find the pKa of X-281, you prepare a 0.082 mol L test solution of X-281. The pH of the solution is determined to be 2.70. What is the pKa of X-281? Express your answer numerically. View Available Hint(s) pKa = Submit
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Given pH 270 H 10 270 19952610 3 M HA ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Statistics And Probability For Engineers
Authors: Douglas C. Montgomery, George C. Runger
6th Edition
1118539710, 978-1118539712
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



