Question
0.65 g of hydrogen chloride (HCI) is dissolved in water to make 4.0L of solution. What is the pH of the resulting hydrochloric acid
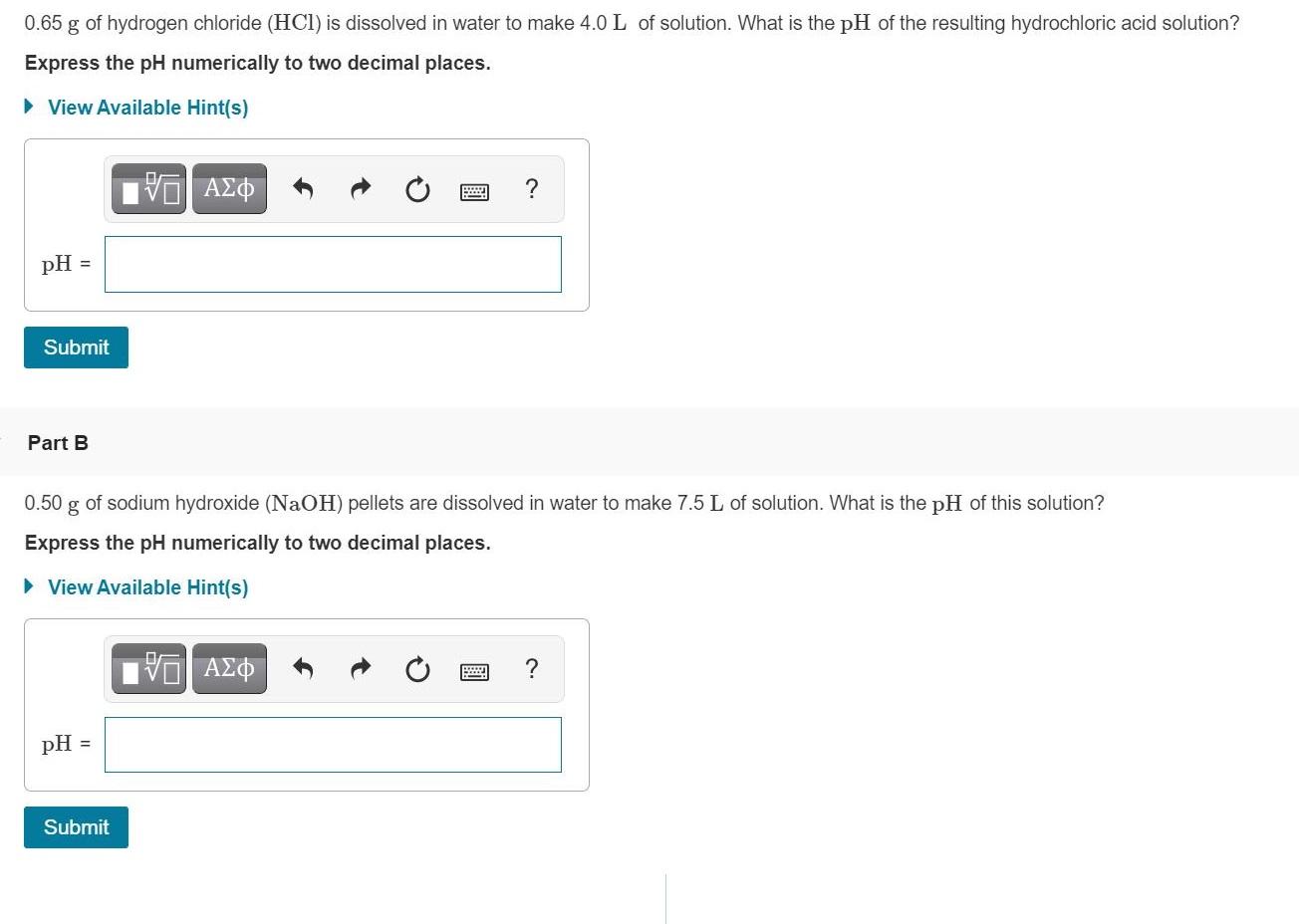
0.65 g of hydrogen chloride (HCI) is dissolved in water to make 4.0L of solution. What is the pH of the resulting hydrochloric acid solution? Express the pH numerically to two decimal places. View Available Hint(s) pH = Submit Part B of sodium hydroxide (NaOH) pellets are dissolved in water to make 7.5 L of solution. What is the pH of this solution? 0.50 g Express the pH numerically to two decimal places. View Available Hint(s) ? pH = Submit
Step by Step Solution
3.32 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



