Answered step by step
Verified Expert Solution
Question
1 Approved Answer
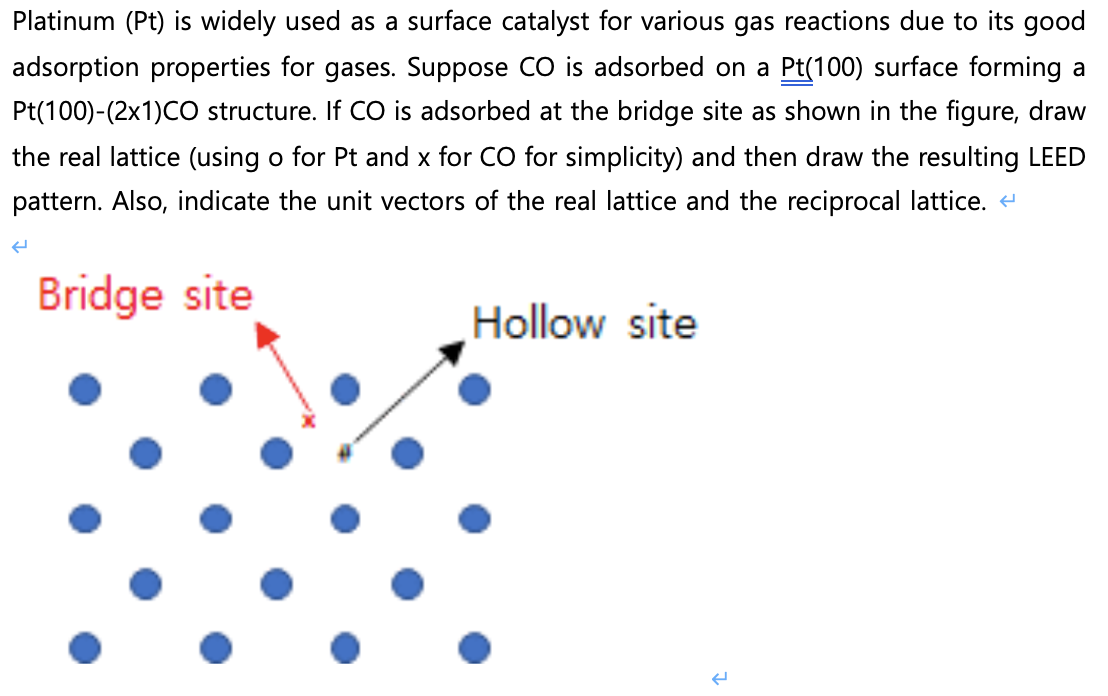
Platinum ( Pt ) is widely used as a surface catalyst for various gas reactions due to its good adsorption properties for gases. Suppose CO
Platinum Pt is widely used as a surface catalyst for various gas reactions due to its good
adsorption properties for gases. Suppose CO is adsorbed on a Pt surface forming a
structure. If is adsorbed at the bridge site as shown in the figure, draw
the real lattice using o for Pt and for for simplicity and then draw the resulting LEED
pattern. Also, indicate the unit vectors of the real lattice and the reciprocal lattice.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


