Answered step by step
Verified Expert Solution
Question
1 Approved Answer
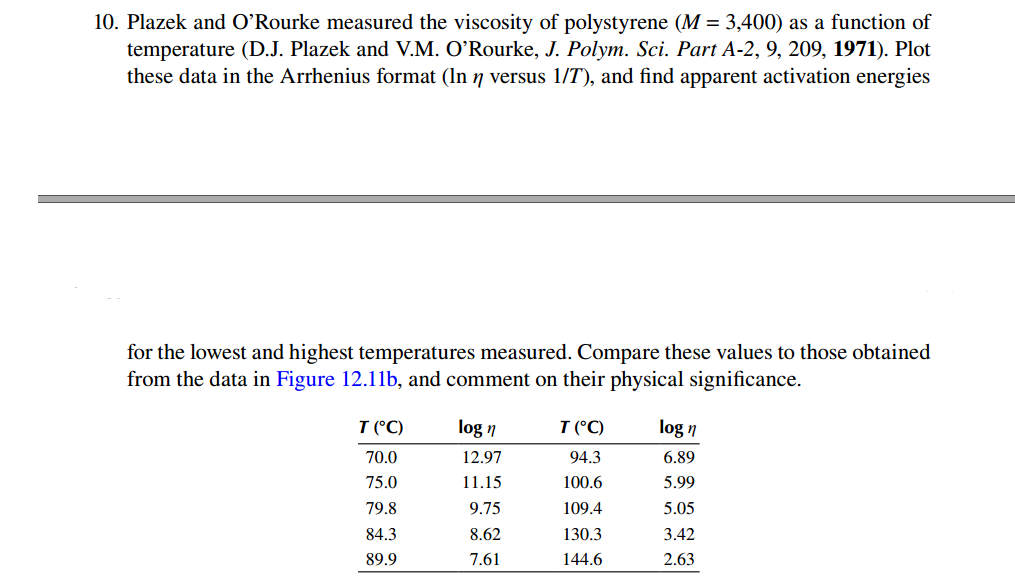
Plazek and O'Rourke measured the viscosity of polystyrene ( M = 3 , 4 0 0 ) as a function of temperature ( D .
Plazek and O'Rourke measured the viscosity of polystyrene as a function of
temperature DJ Plazek and VM O'Rourke, J Polym. Sci. Part A Plot
these data in the Arrhenius format versus and find apparent activation energies
for the lowest and highest temperatures measured. Compare these values to those obtained
from the data in Figure b and comment on their physical significance.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


