pleas the goals and techniques in details



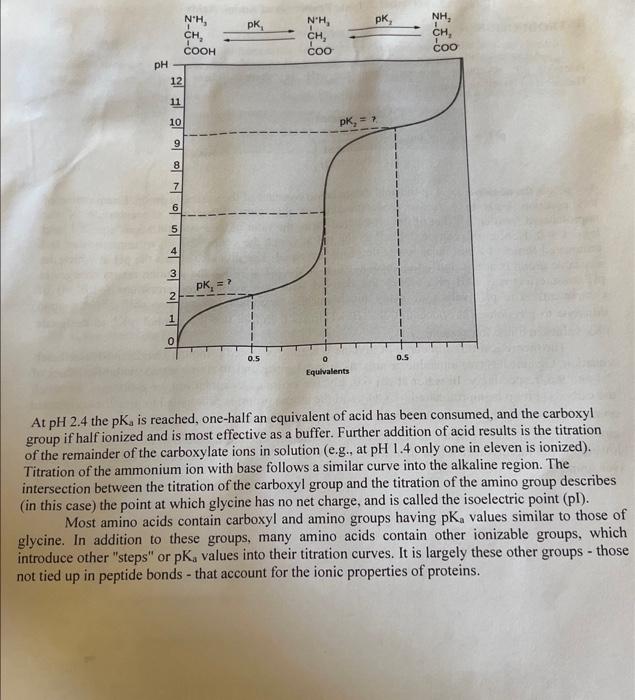
CHEM 2710L Experiment 3: Ionic Properties of Amino Acids Name: Partner Name: Date Lab Performed: 2023-01-31. Introduction What was/were the overall goal(s) of the experiment? ( 2 marks) What techniques/instrumentation were used in this experiment? (2 marks) INTRODUCTION: All amino acids contain ionizable groups that act as weak acids or bases in aqueous solution, releasing or accepting protons when the pH is altered. These ionizations follow the Henderson-Hasselbalch equation: pH=pK3+log10[protonatedform(acid)][unprotonatedform(base)] The pKa is the negative log10 of the acid dissociation constant of the ionizable group. Examination of this equation leads to a further understanding of this term. When the concentration of the unprotonated form equals that of the protonated form, the ratio of their concentrations equals 1 , and log101=0. Hence, pKa can be defined as the pH at which the concentrations of unprotonated and protonated forms of a particular ionizable group are equal. The pK,, also equals the pH at which the ionizable group is at best buffering capacity; that is, the pH at which the solution resists changes in pH most effectively. All pKa values are derived with unit activity concentrations (1.0M) of components. The pKa values of many biochemically important ion systems shift slightly upon dilutions to physiological concentrations. Biochemists therefore use the symbol pK, to designate pKa values in dilute ion systems. The theoretical titration curve of glycine is readily obtained using the Henderson-Hasselbalch equation. Glycine has two ionizable groups: a carboxyl group and an amino group, with pKa values of 2.4 and 9.6, respectively. In water at pH6.0, glycine exists as a dipolar ion, or zwitterion, in which the carboxyl group is unprotonated (COO)and the amino group is protonated to give the substitutec ammonium ion (NH3+). The concentration of the protonated and unprotonated forms of each ion any pH can be calculated from the Henderson-Hasselbalch equation. Addition of acid to the solutio lowers the pH rapidly at first and then more slowly as the buffering action of the carboxyl is exerte (see graph). At pH 2.4 the pKa is reached, one-half an equivalent of acid has been consumed, and the carboxyl group if half ionized and is most effective as a buffer. Further addition of acid results is the titration of the remainder of the carboxylate ions in solution (e.g., at pH 1.4 only one in eleven is ionized). Titration of the ammonium ion with base follows a similar curve into the alkaline region. The intersection between the titration of the carboxyl group and the titration of the amino group describes (in this case) the point at which glycine has no net charge, and is called the isoelectric point (pl). Most amino acids contain carboxyl and amino groups having pKa values similar to those of glycine. In addition to these groups, many amino acids contain other ionizable groups, which introduce other "steps" or pKa values into their titration curves. It is largely these other groups - thos not tied up in peptide bonds - that account for the ionic properties of proteins. EXPERIMENTAL PROCEDURE: Materials Unknowns in solid form 1.00MNaOH 1.00MHCl pH meter 10-mL burette Burette holder Standard buffers Titration of Amino Acid Unknowns NOTE: To ensure consistency, use the same source of deionized water throughout the experiment Overall Procedure: 1).Leam proper use of pH meter. standardize pH meter using 2 buffer standards. 2) Titrate an unknown amino acid using acid 3) Titrate water using acid 4) Titrate SAME unknown amino acid (new solution) using base 5) Titrate water using base Using standard buffers, familiarize yourself with the operation of the pH meter and standardize the instrument. You will be given an unknown sample, record the NUMBER. The sample may be any of the common amino acids in any ionic form, such as zwitterionic form, free base form, or amino acid hydrochloride. Weigh about 400mg of the unknown directly into a dry 50mL beaker, recording the mass to the nearest milligram, and dissolve it in 20mL (accurate!) of distilled water. Set up the electrodes of the pH meter and the burette so that the sample can be titrated while stirring manually with the electrode. Take extreme care to see that the electrodes are not damaged by rough treatment. EXAMINE THE BURET to ensure you can measure the small volume increments properly! Titrate the dissolved unknown sample with 0.5mL aliquots of 1.0MHCl, recording the burette readings and the pH after each addition throughout the titration until the solution approaches pH1.0. Make sure to carefully mix after each addition of acid prior to taking the reading. CLEAN the beaker EXTREMELY well (lots of rinses with tap water. THEN a couple final rinses with distilled water). Titrate another 20mL water sample (a water blank) with 1.0MHCl until the solution reaches pH1.0 (add the HCl in 0.1mL aliquots for the first 1.5mL s and then in 0.5mL portions). Record the pH and volume of acid after each addition after mixing. LABORATORY DATA: Should be presented in your lab BOOK NOT this manual Titration of amino acid solution with HCl : Mass of amino acid in mg Titration of water with HCl : - Table as above Titration of amino acid solution with NaOH : mass of amino acid in mg Titration of water with NaOH : - Table as above In your report include a plot of pH vs milliequivalents (i.e., mmol) of HCl and NaOH consumed by sample alone (use the data in the correction tables). From your corrected titration curves obtain the following: Milliequivalents of HCl to reach endpoint (if possible) pK values Milliequivalents of NaOH to reach endpoint pK values Molar mass of amino acid (mg sample/milliequivalents): Identity of amino acid Lab Report: See marking scheme information on ACORN and Prelab Lecture Notes