Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please and thank you! The graph represents the titration of an amino acid with NaOH solution 12-1 11 10 PK, -9,69 9 8 7. pl
please and thank you! 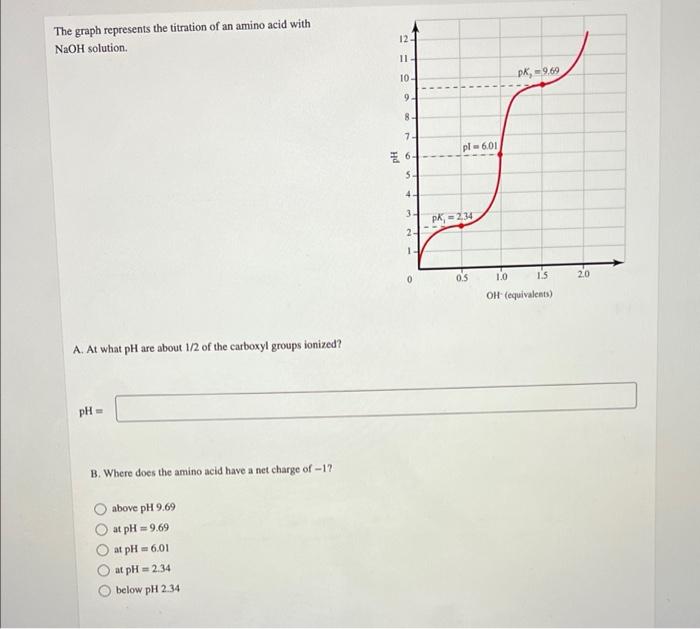
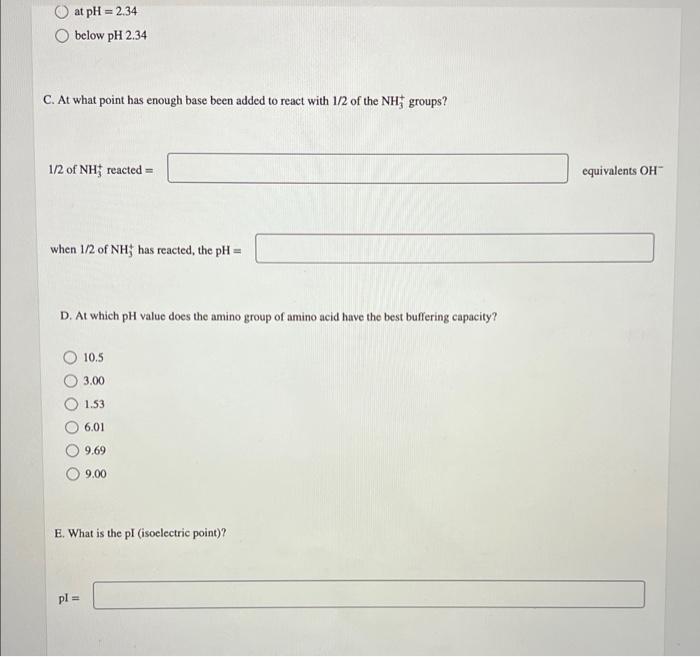

The graph represents the titration of an amino acid with NaOH solution 12-1 11 10 PK, -9,69 9 8 7. pl -601 26. SE pk = 234 2- 11 05 20 1.0 1.5 OH (equivalents) A. At what pH are about 1/2 of the carboxyl groups ionized? pH B. Where does the amino acid have a net charge of -1? above pH 9.69 at pH = 9.69 at pH 6.01 at pH = 234 below pH 2.34 at pH = 2.34 below pH 2.34 C. At what point has enough base been added to react with 1/2 of the NH groups? 1/2 of NH; reacted = equivalents OH when 1/2 of NH; has reacted, the pH = D. At which pH value does the amino group of amino acid have the best buffering capacity? 10.5 3.00 1.53 6.01 9.69 9.00 E. What is the pl (isoelectric point)? pl = The pK, of the a-carboxyl group of serine is 2.21, and the pK, of its a-amino group is 9.15. Calculate the average net charge on serine if it is in a solution that has a pH of 8.50. 0 average net charge on serine: Incorrect 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


