Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer 1. An initial rate experiment was performed to determine the rate law of: 1BrO31+5Br1+6H+3Br2+3H2O The data that was collected is as follows: a.
please answer 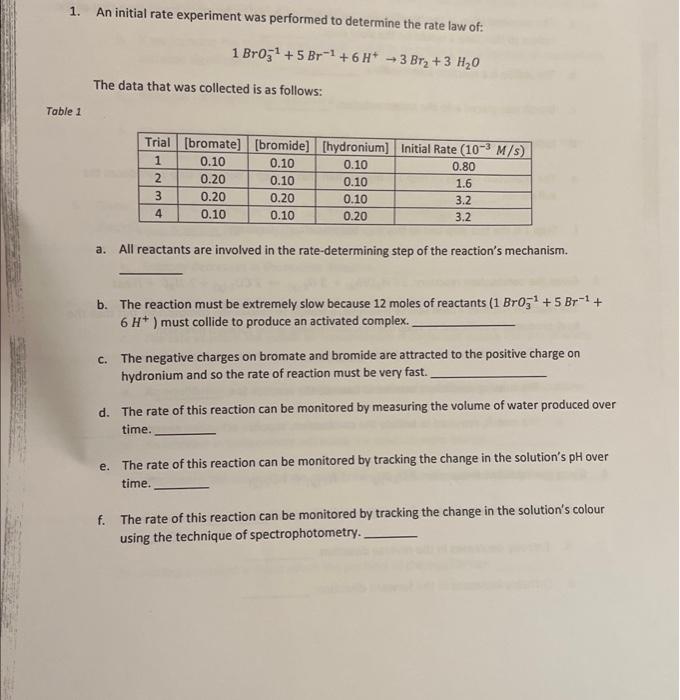
1. An initial rate experiment was performed to determine the rate law of: 1BrO31+5Br1+6H+3Br2+3H2O The data that was collected is as follows: a. All reactants are involved in the rate-determining step of the reaction's mechanism. b. The reaction must be extremely slow because 12 moles of reactants (1BrO31+5Br1+ 6H+) must collide to produce an activated complex. c. The negative charges on bromate and bromide are attracted to the positive charge on hydronium and so the rate of reaction must be very fast. d. The rate of this reaction can be monitored by measuring the volume of water produced over time. e. The rate of this reaction can be monitored by tracking the change in the solution's pH over time. f. The rate of this reaction can be monitored by tracking the change in the solution's colour using the technique of spectrophotometry 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


